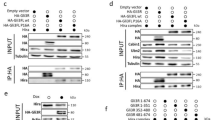
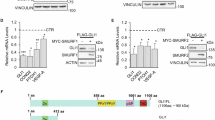
Abstract
Hedgehog signalling is crucial for development and is deregulated in several tumours, including medulloblastoma. Regulation of the transcriptional activity of Gli (glioma-associated oncogene) proteins, effectors of the Hedgehog pathway, is poorly understood. We show here that Gli1 and Gli2 are acetylated proteins and that their HDAC-mediated deacetylation promotes transcriptional activation and sustains a positive autoregulatory loop through Hedgehog-induced upregulation of HDAC1. This mechanism is turned off by HDAC1 degradation through an E3 ubiquitin ligase complex formed by Cullin3 and REN, a Gli antagonist lost in human medulloblastoma. Whereas high HDAC1 and low REN expression in neural progenitors and medulloblastomas correlates with active Hedgehog signalling, loss of HDAC activity suppresses Hedgehog-dependent growth of neural progenitors and tumour cells. Consistent with this, abrogation of Gli1 acetylation enhances cellular proliferation and transformation. These data identify an integrated HDAC- and ubiquitin-mediated circuitry, where acetylation of Gli proteins functions as an unexpected key transcriptional checkpoint of Hedgehog signalling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ruitz i Altaba, A. Hedgehog-Gli Signaling in Human Diseases (Plenum, 2006).
Jiang, J. & Hui, C. C. Hedgehog signaling in development and cancer. Dev. Cell 15, 801–812 (2008).
Ruppert, J. M., Vogelstein, B. & Kinzler, K. W. The zinc finger protein GLI transforms primary cells in cooperation with adenovirus E1A. Mol. Cell Biol. 11, 1724–1728 (1991).
Kimura, H., Stephen, D., Joyner, A. & Curran, T. Gli1 is important for medulloblastoma formation in Ptc1+/− mice. Oncogene 24, 4026–4036 (2005).
Ferretti, E. et al. Concerted microRNA control of Hedgehog signalling in cerebellar neuronal progenitor and tumour cells. EMBO J. 27, 2616–2627 (2008).
Huntzicker, E. G. et al. Dual degradation signals control Gli protein stability and tumor formation. Genes Dev. 20, 276–281 (2006).
Di Marcotullio, L. et al. Numb is a suppressor of Hedgehog signalling and targets Gli1 for Itch-dependent ubiquitination. Nature Cell Biol. 8, 1415–1423 (2006).
Minucci, S. & Pelicci, P. G. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nature Rev. Cancer 6, 38–51 (2006).
Glozak, M. A., Sengupta, N., Zhang, X. & Seto, E. Acetylation and deacetylation of non-histone proteins. Gene 363, 15–23 (2005).
Zupkovitz, G. et al. Negative and positive regulation of gene expression by mouse histone deacetylase 1. Mol. Cell Biol. 26, 7913–7928 (2006).
Yang, X. J. & Seto, E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nature Rev. Mol. Cell Biol. 9, 206–218 (2008).
Dai, P. et al. Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J. Biol. Chem. 274, 8143–8152 (1999).
Dai, P. et al. Ski is involved in transcriptional regulation by the repressor and full-length forms of Gli3. Genes Dev. 16, 2843–2848 (2002).
Yoon, J. W. et al. GLI activates transcription through a herpes simplex viral protein 16-like activation domain. J. Biol. Chem. 273, 3496–3501 (1998).
Di Marcotullio, L. et al. REN(KCTD11) is a suppressor of Hedgehog signaling and is deleted in human medulloblastoma. Proc. Natl Acad. Sci. USA 101, 10833–10838 (2004).
Taipale, J. et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature 406, 1005–1009 (2000).
Chen, J. K., Taipale, J., Young, K. E., Maiti, T. & Beachy, P. A. Small molecule modulation of Smoothened activity. Proc. Natl Acad. Sci. USA 99, 14071–14076 (2002).
Canettieri, G. et al. The coactivator CRTC1 promotes cell proliferation and transformation via AP-1. Proc. Natl Acad. Sci. USA 106, 1445–1450 (2009).
Cheng, S. Y. & Bishop, J. M. Suppressor of Fused represses Gli-mediated transcription by recruiting the SAP18-mSin3 corepressor complex. Proc. Natl Acad. Sci. USA 99, 5442–5447 (2002).
Argenti, B. et al. Hedgehog antagonist REN(KCTD11) regulates proliferation and apoptosis of developing granule cell progenitors. J. Neurosci. 25, 8338–8346 (2005).
Goodrich, L. V., MilenkoviĆ, L., Higgins, K. M. & Scott, M. P. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277, 1109–1113 (1997).
Pintard, L., Willems, A. & Peter, M. Cullin-based ubiquitin ligases: Cul3–BTB complexes join the family. EMBO J. 23, 1681–1687 (2004).
Bar, E. E., Chaudhry, A., Farah, M. H. & Eberhart, C. G. Hedgehog signaling promotes medulloblastoma survival via Bc/II. Am. J. Pathol. 170, 347–355 (2007).
Montgomery, R. L., Hsieh, J., Barbosa, A. C., Richardson, J. A. & Olson, E. N. Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proc. Natl Acad. Sci. USA 106, 7876–7881 (2009).
Shakéd, M. et al. Histone deacetylases control neurogenesis in embryonic brain by inhibition of BMP2/4 signaling. PLoS One 3, e2668 (2008).
Zhao, H., Ayrault, O., Zindy, F., Kim, J. H. & Roussel, M. F. Post-transcriptional down-regulation of Atoh1/Math1 by bone morphogenic proteins suppresses medulloblastoma development. Genes Dev. 22, 722–727 (2008).
Rios, I., Alvarez-Rodriguez, R., Martí, E. & Pons, S. Bmp2 antagonizes sonic hedgehog-mediated proliferation of cerebellar granule neurones through Smad5 signalling. Development 131, 3159–3168 (2004).
Cunliffe, V. T. Histone deacetylase 1 is required to repress Notch target gene expression during zebrafish neurogenesis and to maintain the production of motoneurones in response to hedgehog signalling. Development 131, 2983–2995 (2004).
Kim, J. E., Chen, J. & Lou, Z. DBC1 is a negative regulator of SIRT1. Nature 451, 583–586 (2008).
Zhao, W. et al. Negative regulation of the deacetylase SIRT1 by DBC1. Nature 451, 587–590 (2008).
Spiller, S. E., Ravanpay, A. C., Hahn, A. W. & Olson, J. M. Suberoylanilide hydroxamic acid is effective in preclinical studies of medulloblastoma. J. Neurooncol. 79, 259–270 (2006).
Ecke, I. et al. Antitumor effects of a combined 5-aza-2′deoxycytidine and valproic acid treatment on rhabdomyosarcoma and medulloblastoma in Ptch mutant mice. Cancer Res. 69, 887–895 (2009).
Scales, S. J. & de Sauvage, F. J. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol. Sci. 30, 303–312 (2009).
Chun, S. G., Zhou, W. & Yee, N. S. Combined targeting of histone deacetylases and hedgehog signaling enhances cytoxicity in pancreatic cancer. Cancer Biol. Ther. 8, 1328–1339 (2009).
Canettieri, G. et al. Attenuation of a phosphorylation-dependent activator by an HDAC–PP1 complex. Nature Struct. Biol. 10, 175–181 (2003).
Zhang, Q. et al. A hedgehog-induced BTB protein modulates hedgehog signaling by degrading Ci/Gli transcription factor. Dev. Cell 10, 719–729 (2006).
Peschiaroli, A. et al. SCFβTrCP-mediated degradation of Claspin regulates recovery from the DNA replication checkpoint response. Mol. Cell 23, 319–329 (2006).
Gu, W. & Roeder, R. G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90, 595–606 (1997).
Zhao, X. et al. The HECT-domain ubiquitin ligase Huwe1 controls neural differentiation and proliferation by destabilizing the N-Myc oncoprotein. Nature Cell Biol. 10, 643–653 (2008).
Lahm, A. et al. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc. Natl Acad. Sci. USA 104, 17335–17340 (2007).
Pappin, D. J. Peptide mass fingerprinting using MALDI-TOF mass spectrometry. Methods Mol. Biol. 211, 211–219 (2003).
Zheng, N. et al. Structure of the Cul1–Rbx1–Skp1–F boxSkp2 SCF ubiquitin ligase complex. Nature 416, 703–709 (2002).
Acknowledgements
We thank M. P. Scott for the gift of Ptch1−/− cells, G. Giannini and M. Levrero, for helpful suggestions, L. Di Magno, M. Della Guardia, C. Fragomeli and D. Mazzà for experimental support. This work was supported by the Associazione Italiana per la Ricerca sul Cancro, Telethon Grant GGP07118, the Ministry of University and Research (FIRB and PRIN), the Ministry of Health, the Fondazione Roma Foundation, the Mariani Foundation, the Cenci-Bolognetti Foundation and the Rome Oncogenomic Center.
Author information
Authors and Affiliations
Contributions
G.C. and L.D.M. designed and performed experiments, analysed data and wrote the paper; A.G., S.C., L.A., P.I., L.P., E.M., M.P., G.D.S., E.M.P., P.G. and A.G. performed experiments; E.D.S., E.F., C.S., L.V., C.P., M.E.S. and I.S. analysed data; A.G. designed experiments, analysed data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information Figures (PDF 1387 kb)
Rights and permissions
About this article
Cite this article
Canettieri, G., Di Marcotullio, L., Greco, A. et al. Histone deacetylase and Cullin3–RENKCTD11 ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylation. Nat Cell Biol 12, 132–142 (2010). https://doi.org/10.1038/ncb2013
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2013