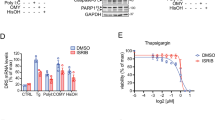
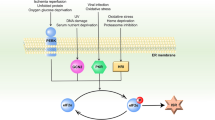
Abstract
When confronted with environmental stress, cells either activate defence mechanisms to survive, or initiate apoptosis, depending on the type of stress. Certain types of stress, such as hypoxia, heatshock and arsenite (type 1 stress), induce cells to assemble cytoplasmic stress granules (SGs), a major adaptive defence mechanism. SGs are multimolecular aggregates of stalled translation pre-initiation complexes that prevent the accumulation of mis-folded proteins1. Type 2 stress, which includes X-rays and genotoxic drugs, induce apoptosis through the stress-activated p38 and JNK MAPK (SAPK) pathways. A functional relationship between the SG and SAPK responses is unknown. Here, we report that SG formation negatively regulates the SAPK apoptotic response, and that the signalling scaffold protein RACK1 functions as a mediator between the two responses. RACK1 binds to the stress-responsive MTK1 MAPKKK and facilitates its activation by type 2 stress; however, under conditions of type 1 stress, RACK1 is sequestered into SGs. Thus, type 1 conditions suppress activation of the MTK1–SAPK pathway and apoptosis induced by type 2 stress. These findings may be relevant to the problem of hypoxia-induced resistance to cancer chemotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
18 November 2008
In the version of this article initially published online, the last paragraph before Methods, incorrectly read "...type 2 stress suppresses the apoptosis induced by type 1 stress, by..." This sentence should read "...type 1 stress suppresses the apoptosis induced by type 2 stress, by...". This error has been corrected in the HTML and PDF versions of the article.
References
Anderson, P. & Kedersha, N. RNA granules. J. Cell Biol. 172, 803–808 (2006).
Tourriere, H. et al. The RasGAP-associated endoribonuclease G3BP asembles stress granules. J. Cell Biol. 160, 823–831 (2003).
Kedersha, N. et al. Evidence that ternary complex (eIF2–GTP-tRNAiMet)-defective preinitiation complexs are core constituents of mammalian stress granules. Mol. Biol. Cell 13, 195–210 (2002).
Kim, W. J., Back, S. H., Kim, V., Ryu, I. & Jang, S. K. Sequestration of TRAF2 into stress granules interrupts tumor necrosis factor signaling under stress conditions. Mol. Cell. Biol. 25, 2450–2462 (2005).
Deigendesch, N., Koch-Nolte, F. & Rothenburg, S. ZBP1 subcellular localization and association with stress granules is controlled by its Z-DNA binding domain. Nucleic Acids Res. 34, 5007–5020 (2006).
Chen, Z. et al. MAP kinases. Chem. Rev. 101, 2449–2476 (2001).
Takekawa, M., Posas, F. & Saito, H. A human homolog of the yeast Ssk2/Ssk22 MAP kinase kinase kinases, MTK1, mediates stress-induced activation of the p38 and JNK pathways. EMBO J. 16, 4973–4982 (1997).
Gerwins, P., Blank, J. L. & Johnson, G. L. Cloning of a novel mitogen-activated protein kinase kinase kinase, MEKK4, that selectively regulates the c-Jun amino terminal kinase pathway. J. Biol. Chem. 272, 8288–8295 (1997).
Ichijo, H. et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275, 90–94 (1997).
Yamaguchi, K. et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science 270, 2008–2011 (1995).
Takekawa, M. & Saito, H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 95, 521–530 (1998).
Mita, H., Tsutsui, J., Takekawa, M., Witten, E. A. & Saito, H. Regulation of MTK1/MEKK4 kinase activity by its N-terminal autoinhibitory domain and GADD45 binding. Mol. Cell. Biol. 22, 4544–4555 (2002).
Miyake, Z., Takekawa, M., Ge, Q. & Saito, H. Activation of MTK1/MEKK4 by GADD45 through induced N–C dissociation and dimerization-mediated trans autophosphorylation of the MTK1 kinase domain. Mol. Cell. Biol. 27, 2765–2776 (2007).
Fornace, A. J. Jr., Alamo, I. Jr. & Hollander, M. C. DNA damage-inducible transcripts in mammalian cells. Proc. Natl Acad. Sci. USA 85, 8800–8804 (1988).
Lievermann, D. A. & Hoffman, B. Gadd45 in the response of hematopoietic cells to genotoxic stress. Blood Cells Mol. Dis. 39, 329–335 (2007).
Takekawa, M., Tatebayashi, K. & Saito, H. Conserved docking site is essential for activation of mammalian MAP kinase kinases by specific MAP kinase kinase kinases. Mol. Cell 18, 295–306 (2005).
Ron, D. et al. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc. Natl Acad. Sci. USA 91, 839–843 (1994).
Nilsson, J., Sengupta, J., Frank, J. & Nissen, P. Regulation of eukaryotic translation by the RACK1 protein: a platform for signalling molecules on the ribosome. EMBO Rep. 5, 1137–1141 (2004).
Lopez-Bergami, P. et al. RACK1 mediates activation of JNK by protein kinase C. Mol. Cell 19, 309–320 (2005).
Shibuya, H. et al. TAB1: an activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science 272, 1179–1182 (1996).
Kimball, S. R., Horetsky, R. L., Ron, D., Jefferson, L. S. & Harding, H. P. Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am. J. Physiol. Cell Physiol. 284, 273–284 (2002).
Kedersha, N. L., Gupta, M., Li, W., Miller, I. & Anderson, P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2α to the assembly of mammalian stress granules. J. Cell Biol. 147, 1431–1441 (1999).
Tian, Q., Streuli, M., Saito, H., Schlossman, S. F. & Anderson, P. A polyadenylate binding protein localized to the granules of cytolytic lmphocytes induces DNA fragmentation in target cells. Cell 67, 629–639 (1991).
Sengupta, J. et al. Identification of the versatile scaffold protein RACK 1 on the eukaryotic ribosome by cryo-EM. Nature Struct. Mol. Biol. 11, 957–962 (2004).
Kayali, F., Montie, H. L., Rafols, J. A. & DeGracia, D. J. Prolonged translation arrest in reperfused hippocampal cornu Ammonis 1 is mediated by stress granules. Neuroscience 134, 1223–1245 (2005).
Moeller, B. J., Cao, Y., Li, C. Y. & Dewhirst, M. W. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 5, 429–441 (2004).
Harris, A. L. Hypoxia - a key regulatory factor in tumour growth. Nature Rev. Cancer 2, 38–47 (2002).
Brown, J. M. & Wilson, W. R. Exploiting tumour hypoxia in cancer treatment. Nature Rev. Cancer 4, 437–447 (2004).
Takekawa, M., Maeda, T. & Saito, H. Protein phosphatase 2Cα inhibits the human stress-responsive p38 and JNK MAPK pathways. EMBO J. 17, 4744–4752 (1998).
Chi, H., Lu, B., Takekawa, M., Davis, R. J. & Flavell, R. A. GADD45β/GADD45γ and MEKK4 comprise a genetic pathway mediating STAT4-independent IFNγ production in T cells. EMBO J. 23, 1576–1586 (2004).
Acknowledgements
This work was supported in part by several grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (M.T. and H.S.), and by a PRESTO programme from the Japan Science and Technology Agency (M.T.). We thank P. O'Grady for critical reading of the manuscript and T. Chano (Shiga University of Medical Science), S. Iwata (University of Tokyo), K. Matsumoto (Nagoya University) and H. Ichijo (University of Tokyo) for plasmids.
Author information
Authors and Affiliations
Contributions
K.A. and M.T. designed and performed the experiments; H.F. and S.I.-O. performed the mass spectrometry analyses; K.A., M.T. and H.S. analysed the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1920 kb)
Rights and permissions
About this article
Cite this article
Arimoto, K., Fukuda, H., Imajoh-Ohmi, S. et al. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat Cell Biol 10, 1324–1332 (2008). https://doi.org/10.1038/ncb1791
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1791