Abstract
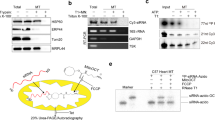
RNA interference (RNAi) is a powerful tool used to manipulate gene expression or determine gene function1,2. One technique of expressing the short double-stranded (ds) RNA intermediates required for interference in mammalian systems is the introduction of short-interfering (si) RNAs3,4,5,6. Although RNAi strategies are reliant on a high degree of specificity, little attention has been given to the potential non-specific effects that might be induced. Here, we found that transfection of siRNAs results in interferon (IFN)-mediated activation of the Jak–Stat pathway and global upregulation of IFN-stimulated genes. This effect is mediated by the dsRNA-dependent protein kinase, PKR, which is activated by 21-base-pair (bp) siRNAs and required for upregulation of IFN-β in response to siRNAs. In addition, we show by using cell lines deficient in specific components mediating IFN action that the RNAi mechanism itself is independent of the interferon system. Thus, siRNAs have broad and complicating effects beyond the selective silencing of target genes when introduced into cells. This is of critical importance, as siRNAs are currently being explored for their potential therapeutic use7,8.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zamore, P.D., Tuschl, T., Sharp, P.A. & Bartel, D.P. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 101, 25–33 (2000).
Hannon, G.J. RNA interference. Nature 418, 244–251 (2002).
Elbashir, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).
Donze, O. & Picard, D. RNA interference in mammalian cells using siRNAs synthesized with T7 RNA polymerase. Nucleic Acids Res. 30, e46 (2002).
Xia, H., Mao, Q., Paulson, H.L. & Davidson, B.L. siRNA-mediated gene silencing in vitro and in vivo. Nature Biotechnol. 20, 1006–1010 (2002).
Hutvagner, G. & Zamore, P.D. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 297, 2056–2060 (2001).
Jacque, J., Triques, K. & Stevenson, M. Modulation of HIV-1 replication by RNA interference. Nature 418, 435–438 (2002).
Gitlin, L., Karelsky, S. & Andino, R. Short interfering RNA confers intracellular antiviral immunity in human cells. Nature 418, 430–434 (2002).
Haque, S.J. & Williams, B.R.G. Signal transduction in the interferon system. Semin. Oncol. 25, 14–22 (1998).
Stark, G.R., Kerr, I.M., Williams, B.R., Silverman, R.H. & Schreiber, R.D. How cells respond to interferons. Annu. Rev. Biochem. 67, 227–264 (1998).
Srivastava, S.P., Kumar, K.U. & Kaufman, R.J. Phosphorylation of eukaryotic translation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J. Biol. Chem. 273, 2416–2423 (1998).
Kumar, A., Haque, J., Lacoste, J., Hiscott, J. & Williams, B.R.G. Double-stranded RNA-dependent protein kinase activates transcription factor NF-κB by phosphorylating IκB. Proc. Natl Acad. Sci. USA 91, 6288–6292 (1994).
Williams, B.R., Gilbert, C.S. & Kerr, I.M. The respective roles of the protein kinase and pppA2′p5′A2′p5 A-activated endonucleases in the inhibition of protein synthesis by double-stranded RNA in rabbit reticulocyte lysates. Nucleic Acids Res. 6, 1335–1350 (1979).
Williams, B.R.G. PKR; a sentinel kinase for cellular stress. Oncogene 18, 6112–6120 (1999).
Silverman, R.H. in Ribonucleases: structure and function (eds G. D'Alessio and J. F. Riordan) Ch. 16, 515–551 (Academic Press, St Louis, 1997).
Pellegrini, S., John, J., Shearer, M., Kerr, I.M. & Stark, G.R. Use of a selectable marker regulated by α interferon to obtain mutations in the signaling pathway. Mol. Cell Biol. 9, 4605–4612 (1989).
Tavernarakis, N., Wang, S.L., Dorovkov, M., Ryazanov, A. & Driscoll, M. Heritable and inducible genetic interference by double-stranded RNA encoded by transgenes. Nature Genet. 24, 180–183 (2000).
Alexopoulou, L., Holt, A.C., Medzhitov, R. & Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Matsumoto, M., Kikkawa, S., Kohase, M., Miyake, K. & Seya, T. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated gene silencing. Biochem. Biophys. Res. Commun. 293, 1364–1369 (2002).
Yang, et al. Deficient signaling in mice devoid of the double-stranded RNA dependent protein kinase, PKR. EMBO J. 14, 6095–6106 (1995).
Zhou, A. et al. Interferon action and apoptosis are defective in mice devoid of 2′-5′-oligoadenylate-dependent RNase L. EMBO J. 16, 3297–3304 (1997).
Zhou, A., Paranjape, J.M., Der, S.D., Williams, B.R.G. & Silverman, R.H. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology 258, 435–440 (1999).
Goh, K.C., Haque, S.J. & Williams, B.R.G. p38 MAP kinase is required for Stat1 serine phosphorylation and transcriptional activation induced by interferons. EMBO J. 18, 5601–5608 (1999).
Acknowledgements
We would like to thank Z. Wang for synthesizing luciferase siRNAs, P. Stanhope-Baker for providing the Supplementary Information on the non-specific effects of the WT-1 siRNAs and A. Sadler for comments on the manuscript. This work was supported by National Institutes of Health (NIH) grants RO1 AI34039 and PO1 CA62220.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information, Fig. S1
Supplementary Information, Fig. S2 (PPT 1722 kb)
Supplementary Information, Fig. S3
Supplementary Information, Fig. S4
Rights and permissions
About this article
Cite this article
Sledz, C., Holko, M., de Veer, M. et al. Activation of the interferon system by short-interfering RNAs. Nat Cell Biol 5, 834–839 (2003). https://doi.org/10.1038/ncb1038
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1038