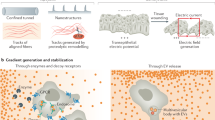
Abstract
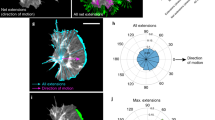
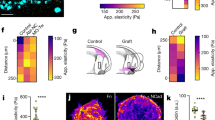
Directed cell migration requires the breaking of cell symmetry to generate a cell front and a cell rear along an axis approximately aligned with the direction of locomotion. In most cell types, regulated actin polymerization promotes initial cell front formation and its subsequent persistent protrusion, whereas myosin II-based forces are required to initially create and then maintain the cell rear. Molecular models for cell migration have focused extensively on cell protrusion, and the breaking of cell symmetry is almost universally portrayed with the cell front forming first. Although data supports this model for cells moving towards chemo-attractants, in the absence of any guidance cue, cell symmetry is broken by the cells constitutively forming the cell rear first. This allows an alternative model for triggering cell migration starting with retraction at the back of the cell. In this model, actomyosin II activity within the cell body and prospective cell rear occurs before a spatial bias in actin polymerization at the cell front. Creating the cell rear first may be a useful tool employed by a wide-range of migrating cell types, particularly when moving away from repellent cues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Lauffenburger, D. A. & Horwitz, A. F. Cell migration: a physically integrated molecular process. Cell 84, 359–369 (1996).
Mitchison, T. J. & Cramer, L. P. Actin-based cell motility and cell locomotion. Cell 84, 371–379 (1996).
Sheetz, M. P., Felsenfeld, D., Galbraith, C. G. & Choquet, D. Cell migration as a five-step cycle. Biochem. Soc. Symp. 65, 233–243 (1999).
Ridley, A. J. Rho GTPases and cell migration. J. Cell Sci. 114, 2713–2722 (2001).
Ridley, A. J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003).
Zigmond, S. H. & Sullivan, S. J. Sensory adaptation of leukocytes to chemotactic peptides. J. Cell Biol. 82, 517–527 (1979).
Wong, K., Pertz, O., Hahn, K. & Bourne, H. Neutrophil polarization: spatiotemporal dynamics of RhoA activity support a self-organizing mechanism. Proc. Natl Acad. Sci. USA 103, 3639–3644 (2006).
Mseka, T., Bamburg, J. R. & Cramer, L. P. ADF/cofilin family proteins control formation of oriented actin-filament bundles in the cell body to trigger fibroblast polarization. J. Cell Sci. 120, 4332–4344 (2007).
Yam, P. T. et al. Actin-myosin network reorganization breaks symmetry at the cell rear to spontaneously initiate polarized cell motility. J. Cell Biol. 178, 1207–1221 (2007).
Chen, W. Surface changes during retraction-induced spreading of fibroblasts. J. Cell Sci. 49, 1–13 (1981).
Dunn, G. A. Mechanisms of fibroblast locomotion, in Cell Adhesion and Motility, 3rd BSCB Symposium (eds Curtis, A. S. G. & Pitts, J. D.) 409–423 (Cambridge Univ. Press, 1980).
Dunn, G. A. & Zicha, D. Dynamics of fibroblast spreading. J. Cell Sci. 108, 1239–1249 (1995).
Verkhovsky, A. B., Svitkina, T. M. & Borisy, G. G. Self-polarization and directional motility of cytoplasm. Curr. Biol. 9, 11–20 (1999).
Mast, S. O. Structure, movement, locomotion, and stimulation in amoeba. J. Morph. Physiol. 41, 347–425 (1926).
Allen, R. D. & Taylor, D. L. The molecular basis of amoeboid movement, in Molecules and Cell Movement (eds. Inoue, S. & Stephens, R. E.) 239–258 (Raven, New York, 1975).
Xu, J. et al. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 114, 201–214 (2003).
Pollard, T. D. & Borisy, G. G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 (2003).
Carlier, M. F. & Pantaloni, D. Control of actin assembly dynamics in cell motility. J. Biol. Chem. 282, 23005–23009 (2007).
Charras, G. & Paluch, E. Blebs lead the way: how to migrate without lamellipodia. Nat. Rev. Mol. Cell Biol. 9, 730–736 (2008).
Niggli, V. Signaling to migration in neutrophils: importance of localised pathways. Intl. J. Biochem. Cell Biol. 35, 1619–1638 (2003).
Koehl, G. & McNally, J. G. Myosin II redistribution during rear retraction and the role of filament assembly and disassembly. Cell Biol. Internatl 26, 287–396 (2002).
Vicente-Manzanares, M. et al. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J. Cell Biol. 176, 573–580 (2007).
Gutjahr, M. C., Rossy, J. & Niggli, V. Role of Rho, Rac, and Rho kinase in phosphorylation of myosin light chain, development of polarity, and spontaneous migration of Walker 256 carcinosarcoma cells. Exp. Cell Res. 308, 422–438 (2005).
Vicente-Manzanares, M. et al. Segregation and activation of myosin IIB creates a rear in migrating cells. J. Cell Biol. 183, 543–554 (2008).
Shutova, M. S., Alexandrova, A. Y. & Vasiliev, J., M. Regulation of polarity in cells devoid of actin bundle system after treatment with inhibitors of myosin II activity. Cell Motil. Cytoskeleton 65, 734–746 (2008).
Lo, C.-M. et al. Nonmuscle Myosin IIB is involved in the guidance of fibroblast migration. Mol. Biol. Cell 15, 982–989 (2004).
Bardi, G., Niggli, V. & Loetscher, P. Rho kinase is required for CCR7-mediated polarization and chemotaxis of T lymphocytes. FEBS Letts. 542, 79–83 (2003).
Cramer, L. P., Siebert, M. & Mitchison, T. J. Identification of novel graded polarity actin filament bundles in locomoting heart fibroblasts: implications for the generation of motile force. J. Cell Biol. 136, 1287–1305 (1997).
Swailes, N. T., Knight, P. J. & Peckham, M. Actin filament organization in aligned prefusion myoblasts. J. Anat. 205, 381–391 (2004).
Svitkina, T. M., Verkhovsky, A. B., McQuade, K. M. & Borisy, G. G. Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J. Cell Biol. 139, 397–415 (1997).
Gardel, M. L. et al. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J. Cell Biol. 183, 999–1005 (2008).
Mseka, T., Coughlin, M. & Cramer, L. P. Graded actin filament polarity is the organization of oriented actomyosin II filament bundles required for fibroblast polarization. Cell Motil. Cytoskel. 66, 743–753 (2009).
Gomes, E. R., Jani, S. & Gundersen, G. G. Nuclear movement regulated by Cdc42, MRCK, myosin and actin flow establishes MTOC polarization in migrating cells. Cell 121, 451–463 (2005).
Zicha, D. et al. Rapid actin transport during cell protrusion. Science 300, 142–145 (2003).
Iwasaki, T. & Wang, Y.-L. Cytoplasmic force gradient in migrating adhesive cells. Biophys. J. 94, L35–L37 (2008).
Keren, K. et al. Intracellular fluid flow in rapidly moving cells. Nat. Cell Biol. 11, 1219–1225 (2009).
Cramer, L. P. Role of actin-filament disassembly in lamellipodium protrusion in motile cells revealed using the drug jasplakinolide. Curr. Biol. 9, 1095–1105 (1999).
Peckham, M. et al. Specific changes to the mechanism of cell locomotion induced by overexpression of β-actin. J. Cell Sci. 114, 1367–1377 (2001).
Blankenship, J. T. et al. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev. Cell 11, 459–470 (2006).
Bertet, C., Sulak, L. & Lecuit, T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429, 667–671 (2004).
Acknowledgements
I thank V. Niggli for helpful discussion and G. Charras, F. Pichaud and T. Mseka for helpful comments on the manuscript, and acknowledge colleagues whose studies were not directly cited because of space restraints.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Table 1 (PDF 176 kb)
Rights and permissions
About this article
Cite this article
Cramer, L. Forming the cell rear first: breaking cell symmetry to trigger directed cell migration. Nat Cell Biol 12, 628–632 (2010). https://doi.org/10.1038/ncb0710-628
Issue Date:
DOI: https://doi.org/10.1038/ncb0710-628
This article is cited by
-
Photo-expansion microscopy enables super-resolution imaging of cells embedded in 3D hydrogels
Nature Materials (2023)
-
Cyclic stretching-induced epithelial cell reorientation is driven by microtubule-modulated transverse extension during the relaxation phase
Scientific Reports (2021)
-
Modelling actin polymerization: the effect on confined cell migration
Biomechanics and Modeling in Mechanobiology (2019)
-
The assembly and function of perinuclear actin cap in migrating cells
Protoplasma (2017)
-
A resilient formin-derived cortical actin meshwork in the rear drives actomyosin-based motility in 2D confinement
Nature Communications (2015)