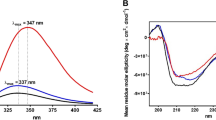
Abstract
Erythrina trypsin/tPA inhibitor (ETI) from the seeds of Erythrina caffra retains its native structure and inhibitory function after reducing its two disulfide bonds. In order to elucidate the specific role of these crosslinks, alanine residues were substituted for cysteines after cloning the gene in Escherichia coll. Expression of the recombinant inhibitor and the substitution mutants, C83A, CC39,83AA, and CC 132,139AA, led to inclusion bodies. After solubilization in guanidinium-chloride (GdmCI)/dithiothreitol and oxidation in glutathione buffer, activity could be recovered at yields up to 80%. The mutant proteins exhibit full inhibitory function without detectable alterations of their native structure. However, their stability is reduced: at acid pH, where the oxidized natural inhibitor retains its native structure, the reduced wildtype protein and the mutants undergo at least partial denaturation, reflected by decreased pH ranges of stability: pH 5–7 for the reduced inhibitor, pH 2.5–8.5 for CC 132,139AA, and pH 3.5–8.5 for C83A and CC39,83AA. Urea and GdmCI denaturation at pH 7 show hysteresis for both the oxidized inhibitor and the double mutant CC132,139AA. In contrast, the reduced protein and the other mutants exhibit true equilibrium transitions at pH 7, with urea half-concentrations of 0.9 M and 1.9 M and GdmCI half-concentrations of 0.5 M and 1.0 M, respectively. The stability of Erythrina trypsin/tPA inhibitor follows the sequence: oxidized ETI > CC 132,139 A A > CC39,83AA and C83A > reduced ETI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Heussen, C., Joubert, F.J. and Dowdle, E.B. 1984. Purification of human tissue plasminogen activator with Erythrina trypsin inhibitors. J. Biol. Chem. 259: 11635–11638.
Zimmerman, M., Quigley, J.P., Ashe, B., Dorn, C., Goldfarb, B. and Troll, W. 1978. Direct fluorescent assay of urokinase and plasminogen activators of normal and malignant cells: Kinetics and inhibitor profiles. Proc. Natl. Acad. Sci. USA 75: 750–753.
Heussen-Schemmer, C., Merrifield, E.H. and Dowdle, E.B., 1991. Erythrina protease inhibitor: Interactions with tissue plasminogen activator. Thrombosis & Haemost. 66: 226–231.
Kohnert, U., Rudolph, R., Verheijen, J.H., Weening-Verhoeff, E.J.D., Stern, A., Opitz, U., Maritn, U., Lill, H., Prinz, H., Lechner, M., Kresse, G., Buckel, P. and Fischer, S. 1992. Biochemical properties of the kringle 2 and protease domains are maintained in the refolded tPA deletion variant BM 06. 022. Prot Engin. 5: 93–100.
Onesti, S., Brick, P. and Blow, D.M. 1991. Crystal structure of a Kunitz-type trypsin inhibitor from Erythrina caffra seeds. J. Mol. Biol. 217: 153–176.
Kim, S.H., Hara, S., Hase, S., Ikenaka, T., Toda, H., Kitamura, K. and Kaizuma, N. 1985. Comparative study on amino acid sequences of Kunitz-type soybean trypsin inhibitors. J. Biochem. 98: 435–448.
Joubert, F.J., Merrifield, E.H. and Dowdle, E.B.D. 1987. The reactive sites of proteinase inhibitors from Erythrinaseeds. Int. J. Biochem. 19: 601–606.
Lehle, K., Wrba, A. and Jaenicke, R. 1994. Erythrina trypsin inhibitor retains its native structure and function after reducing its disulfide groups. J. Mol. Biol. 239: 276–284.
Teixeira, A.V., Dowdle, E.B. and Botes, D.P. 1994. Synthesis and expression of a gene coding for Erythrina trypsin inhibitor (ETI). Biochim. Biophys. Ada 1217: 16–22.
Rudolph, R. 1990. Renaturation of recombinant disulfide-bonded proteins from “inclusion bodies”, pp. 149–171 in Modern Methods in Protein- and Nucleic Acid Research. Tschesche, H. (ed.). de Gruyter Verlag, Berlin, NY.
Joubert, F.J. 1982. Purification and properties of the proteinase inhibitors from Erythrina caffra seed. Int. J. Biochem. 14: 187–193.
Laskowsky, M. 1986. Protein inhibitors of serine proteinases—Mechanism and classification, pp. 1–17 in Nutritional and Toxicological Significance of Enzyme Inhibitors in Foods. Friedman, M. (ed.). Plenum, NY.
Marston, F.O.A. 1986. The purification of eukaryotic polypeptides synthesized in E coli . Biochem. J. 240: 1–12.
Teixeira, A.V., Dowdle, E.B. and Botes, D.P. 1994. Site-directed mutagenesis of the synthetic Erythrina trypsin/tissue plasminogen activator inhibitor encoding-gene to compare the interaction of Erythrina and soybean trypsin inhibitor with tPA. Biochim. Biophys. Ada 1217: 23–28.
Maniatis, T., Fritsch, E.F. and Sambrook, J. 1982. Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory, NY.
Sambrook, J., Fritsch, E.F. and Maniatis, T. 1989. Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Kunkel, T.A., Roberts, J.D. and Zakour, R.A. 1987. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 154: 367–382.
Geisselsoder, J., Witney, R. and Yuckenberg, P. 1987. Efficient site-directed in vitro mutagenesis. BioTechniques 5: 786–791.
Vieira, J. and Messing, J. 1987. Production of single-stranded plasmid DNA. Methods Enzymol. 153: 3–11.
De Boer, H.A., Comstock, L.J., and Basser, M. 1983. The tac promoter: A functional hybrid derived from the trp and lac promoters. Proc. Natl. Acad. Sci. USA 80: 21–25.
Brinkman, U., Mattes, R. and Buckel, P. 1989. High-level expression of recombinant genes in E coli is dependent on the ability of the dnaY gene product. Gene 85: 109–114.
Sanger, F., Micklen, S. and Coulson, A.R. 1977. DNA sequencing with chain termination inhibitors.Proc. Natl. Acad. Sci. USA 74: 5015–5023.
Jaenicke, R. and Rudolph, R. 1989. Folding proteins, p. 191–223. in Protein Structure: A Practical Approach. Creighton, T. E. (ed.). IRL Press, Oxford.
Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of micro-gram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lehle, K., Kohnert, U., Stern, A. et al. Effect of disulfide bonds on the structure, function, and stability of the trypsin/tPA inhibitor from Erythrina caffra: Site-directed mutagenesis, expression, and physiochemical characterization. Nat Biotechnol 14, 476–480 (1996). https://doi.org/10.1038/nbt0496-476
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0496-476
This article is cited by
-
Thermostability of subtilisin nattokinase obtained by site-directed mutagenesis
Wuhan University Journal of Natural Sciences (2014)
-
Stability of Murraya koenigii miraculin-like protein in different physicochemical conditions
Medicinal Chemistry Research (2011)
-
Unexpected pathways to protein stabilization
Nature Biotechnology (1996)