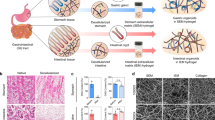
Abstract
Epithelial organoids recapitulate multiple aspects of real organs, making them promising models of organ development, function and disease1,2,3. However, the full potential of organoids in research and therapy has remained unrealized, owing to the poorly defined animal-derived matrices in which they are grown4. Here we used modular synthetic hydrogel networks5,6 to define the key extracellular matrix (ECM) parameters that govern intestinal stem cell (ISC) expansion and organoid formation, and show that separate stages of the process require different mechanical environments and ECM components. In particular, fibronectin-based adhesion was sufficient for ISC survival and proliferation. High matrix stiffness significantly enhanced ISC expansion through a yes-associated protein 1 (YAP)-dependent mechanism. ISC differentiation and organoid formation, on the other hand, required a soft matrix and laminin-based adhesion. We used these insights to build a fully defined culture system for the expansion of mouse and human ISCs. We also produced mechanically dynamic matrices that were initially optimal for ISC expansion and subsequently permissive to differentiation and intestinal organoid formation, thus creating well-defined alternatives to animal-derived matrices for the culture of mouse and human stem-cell-derived organoids. Our approach overcomes multiple limitations of current organoid cultures and greatly expands their applicability in basic and clinical research. The principles presented here can be extended to identify designer matrices that are optimal for long-term culture of other types of stem cells and organoids.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Clevers, H. Modeling development and disease with organoids. Cell 165, 1586–1597 (2016)
Lancaster, M. A. & Knoblich, J. A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125 (2014)
Sasai, Y. Cytosystems dynamics in self-organization of tissue architecture. Nature 493, 318–326 (2013)
Fatehullah, A., Tan, S. H. & Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 18, 246–254 (2016)
Lutolf, M. P., Gilbert, P. M. & Blau, H. M. Designing materials to direct stem-cell fate. Nature 462, 433–441 (2009)
Ranga, A. et al. 3D niche microarrays for systems-level analyses of cell fate. Nat. Commun. 5, 4324 (2014)
Sato, T. & Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340, 1190–1194 (2013)
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009)
Dekkers, J. F. et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 19, 939–945 (2013)
Yui, S. et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat. Med. 18, 618–623 (2012)
Kleinman, H. K. & Martin, G. R. Matrigel: basement membrane matrix with biological activity. Semin. Cancer Biol. 15, 378–386 (2005)
Jabaji, Z. et al. Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium. PLoS One 9, e107814 (2014)
Hughes, C. S., Postovit, L. M. & Lajoie, G. A. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10, 1886–1890 (2010)
Vukicevic, S. et al. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp. Cell Res. 202, 1–8 (1992)
Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 336, 1124–1128 (2012)
Ehrbar, M. et al. Biomolecular hydrogels formed and degraded via site-specific enzymatic reactions. Biomacromolecules 8, 3000–3007 (2007)
Benoit, Y. D., Groulx, J. F., Gagné, D. & Beaulieu, J. F. RGD-dependent epithelial cell-matrix interactions in the human intestinal crypt. J. Signal Transduct. 2012, 248759 (2012)
Simo, P. et al. Changes in the expression of laminin during intestinal development. Development 112, 477–487 (1991)
Simon-Assmann, P. et al. Differential expression of laminin isoforms and α6-β4 integrin subunits in the developing human and mouse intestine. Dev. Dyn. 201, 71–85 (1994)
Wang, F. et al. Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology 145, 383–395 (2013)
Yamamoto, S. et al. Heparan sulfate on intestinal epithelial cells plays a critical role in intestinal crypt homeostasis via Wnt/β-catenin signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G241–G249 (2013)
Fernández-Sánchez, M. E. et al. Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature 523, 92–95 (2015)
Guilak, F. et al. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5, 17–26 (2009)
Gregorieff, A., Liu, Y., Inanlou, M. R., Khomchuk, Y. & Wrana, J. L. Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature 526, 715–718 (2015)
Imajo, M., Ebisuya, M. & Nishida, E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat. Cell Biol. 17, 7–19 (2015)
Aragona, M. et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047–1059 (2013)
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011)
Sternlicht, M. D. & Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 17, 463–516 (2001)
Halder, G., Dupont, S. & Piccolo, S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 13, 591–600 (2012)
Nicodemus, G. D. & Bryant, S. J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. Part B Rev. 14, 149–165 (2008)
Matano, M. et al. Modeling colorectal cancer using CRISPR–Cas9-mediated engineering of human intestinal organoids. Nat. Med. 21, 256–262 (2015)
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772 (2011)
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013)
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015)
Wang, L., Wang, S. & Li, W. RSeQC: quality control of RNA-seq experiments. Bioinformatics 28, 2184–2185 (2012)
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011)
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010)
Law, C. W., Chen, Y., Shi, W. & Smyth, G. K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, R29 (2014)
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015)
Subramanian, A. et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005)
Sadanandam, A. et al. A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat. Med. 19, 619–625 (2013)
Nomizu, M. et al. Identification of cell binding sites in the laminin α1 chain carboxyl-terminal globular domain by systematic screening of synthetic peptides. J. Biol. Chem. 270, 20583–20590 (1995)
Nomizu, M. et al. Cell binding sequences in mouse laminin α1 chain. J. Biol. Chem. 273, 32491–32499 (1998)
Acknowledgements
We thank M. Knobloch (University of Zurich) for helpful discussions, A. Negro (EPFL) for help in the development of PEG–alginate hybrid hydrogels, D. Ossipov (Uppsala University) for providing hyaluronic acid, the Lausanne Genomic Technologies Facility (K. Harshman) for RNA-seq and D. Pioletti (EPFL) for rheometer use. N.G. was supported by an EMBO Long-Term Postdoctoral Fellowship. This work was also supported by funding from the Ecole Polytechnique Fédérale de Lausanne (EPFL). Work performed in the laboratory of H.C. was supported by the NWO Translational Adult Stem Cell Research Grant 40-41400-98-1108 and by a NWO VENI Grant 916.15.182.
Author information
Authors and Affiliations
Contributions
N.G. and M.P.L. conceived the study, designed experiments, analysed data and wrote the manuscript. N.G. was involved in performing and analysing all experiments in the manuscript except for those involving human organoids. P.O.M. helped design experiments and analyse RNA-seq data. A.M. performed qPCR gene expression experiments and analysed data and produced lentiviruses. S.G. performed flow cytometry analysis of integrin expression of ISCs culture in Matrigel and PEG RGD. M.E.B. designed and characterized PEG–alg hydrogel system and helped N.G. perform experiments with ISCs in these matrices. N.S. and H.C. designed experiments and analysed data with human cells. N.S. performed experiments with human cells.
Corresponding author
Ethics declarations
Competing interests
Ecole Polytechnique Fédérale de Lausanne (with M.P.L. and N.G.) has filed patent applications pertaining to synthetic gels for epithelial stem cell and organoid cultures. H.C. is an inventor of several patents on organoid technology.
Additional information
Reviewer Information
Nature thanks L. Li, J. Mills and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Primary crypt culture in synthetic matrices, characterization of ISCs grown in PEG RGD culture with or without CHIR99021 and valproic acid.
a, ISCs cultured in Matrigel and unmodified PEG gels for 24 h. b, ISC colonies formed from freshly isolated mouse intestinal crypts embedded in PEG RGD. c, Relative mRNA levels of intestinal genes, quantified by qPCR. d, ISC colonies cultured under self-renewal conditions in Matrigel, but not PEG gels, contain lysozyme-expressing Paneth cells. e, Stiffness-dependent colony formation and quantification of ISCs cultured with EGF, noggin, R-spondin and Wnt3a. Graphs show individual data points derived from n = 3 independent experiments and means. Scale bars, 50 μm.
Extended Data Figure 2 Effect of controlled matrix softening on ISC colony morphology, YAP activity and growth.
a, Mechanical properties of control and softened PEG alginate (PEG–alg) hybrid gels obtained by selective degradation of the alginate network. Graph shows individual data points derived from 3 independently prepared gels. b, Morphology of day 1 colonies in control and softened PEG–alg gels. c, d, Distribution of YAP in day 1 colonies in control and softened PEG–alg gels (c) and quantification (d). n = 28 colonies (control) and n = 31 (softened). Data are represented as mean ± s.e.m. e, Alginate lyase treatment does not affect colony growth in PEG RGD gels. Softening of PEG–alg gels by alginate-lyase-mediated digestion blocks colony growth. f, Quantification of shRNA-mediated knockdown of YAP. Graph shows individual data points derived from 2 independent experiments and means. g, Effect of verteporfin on ISC colony formation in PEG RGD. Graph shows individual data points derived from 3 independent experiments and means.*P < 0.05; **P < 0.01; ***P < 0.01. Scale bars, 50 μm.
Extended Data Figure 3 Stiff degradable matrices induce an inflammation-like state in ISCs.
a, Gene set enrichment analysis (GSEA) comparing RNA-seq gene expression data of ISCs cultured in degradable compared to non-degradable matrices to published gene signatures (see Methods for details). b, Functional annotation of signalling pathway significantly upregulated in degradable matrices (details and statistics shown in Extended Data Tables 2, 3).
Extended Data Figure 4 Comparison of organoid formation and YAP activity in Matrigel and PEG RGD.
a, Organoid formation does not occur within synthetic PEG RGD matrices. ISC colonies cultured in non-degradable (PEG N-DG) and degradable (PEG DG) PEG RGD matrices, with or without GM6001 for 4 d, and subsequently cultured under organoid formation conditions for 2 d. b, Time-course analysis of morphology and Lgr5–eGFP expression during organoid formation. ISC colonies formed in Matrigel and PEG hydrogels were monitored for 48 h, following a switch to differentiation and organoid formation conditions. c, Localization of YAP at day 1 and day 4 in ISC colonies formed in 300 Pa and 1.3 kPa PEG RGD gels. d, Quantification of nuclear translocation of YAP as a function of time in 300 Pa, 1.3 kPa PEG RGD and Matrigel. Data are shown as means ± s.e.m. To assess the extent of YAP nuclear translocation, n = 21 (day 1, 1.3 kPa); 27 (day 2, 1.3 kPa); 22 (day 3, 1.3 kPa); 22 (day 4, 1.3 kPa); 27 (day 1, 300 Pa); 6 (day 2, 300 Pa); 23 (day 3, 300 Pa); 22 (day 4, 300 Pa); 30 (day 1, Matrigel); 28 (day 2, Matrigel); 30 (day 3, Matrigel); 30 (day 4, Matrigel). *P < 0.05; **P < 0.01; ***P < 0.001. Scale bars, 50 μm.
Extended Data Figure 5 Effect of laminin-derived peptides on organoid formation, and human organoid expansion in PEG RGD.
a, Effect of different laminin-111-derived sequences on intestinal organoid viability. b, Morphology of intestinal organoids grown in Matrigel, plain PEG or PEG-AG73 gels. c, AG73-conjugated PEG matrices significantly enhance the growth of intestinal organoids. d, Effect of AG73 on intestinal organoid viability and growth is concentration-dependent. e, Quantification of intestinal organoid viability in Matrigel, TG PEG-AG73 and MT PEG-AG73. f, Morphology and Lgr5–eGFP expression in intestinal organoids grown in Matrigel and MT PEG-AG73 gels. g, Quantification of Lgr5–eGFP expression in intestinal organoids expanded in Matrigel, TG PEG-AG73 and MT PEG-AG73. h, Establishment of apicobasal polarity and presence of Paneth (lysozyme) cells within organoids grown in MT PEG-AG73. i, AG73 peptides are not capable of supporting differentiation and organoid formation from ISC colonies, whereas full-length laminin-111 is. j, Quantification of nuclear translocation of YAP as a function of time in cells cultiured within mechanically stable and softening gels. Data are shown as means ± s.e.m. To assess the extent of YAP nuclear translocation, n = 21 (day 1, 100% sPEG), 27 (day 2, 100% sPEG), 22 (day 3, 100% sPEG), 22 (day 4, 100% sPEG), 30 (day 1, 75% dPEG), 30 (day 2, 75% dPEG), 30 (day 3, 75% dPEG), and 30 (day 4, 75% dPEG) colonies were analysed. k, l, Phase contrast images (k) and Ki67 expression (l) of human ISC colonies and human patient-derived colorectal cancer organoids grown in PEG RGD. Graphs show individual data points derived from n = 2 (a), n = 3 (c–e, g) independent experiments and means. *P < 0.05; **P < 0.01; ***P < 0.001. Scale bars, 50 μm.
Rights and permissions
About this article
Cite this article
Gjorevski, N., Sachs, N., Manfrin, A. et al. Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560–564 (2016). https://doi.org/10.1038/nature20168
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature20168
This article is cited by
-
Establishment of a chicken intestinal organoid culture system to assess deoxynivalenol-induced damage of the intestinal barrier function
Journal of Animal Science and Biotechnology (2024)
-
The importance of 3D fibre architecture in cancer and implications for biomaterial model design
Nature Reviews Cancer (2024)
-
NAD+ dependent UPRmt activation underlies intestinal aging caused by mitochondrial DNA mutations
Nature Communications (2024)
-
Trends in mechanobiology guided tissue engineering and tools to study cell-substrate interactions: a brief review
Biomaterials Research (2023)
-
Mass production of lumenogenic human embryoid bodies and functional cardiospheres using in-air-generated microcapsules
Nature Communications (2023)