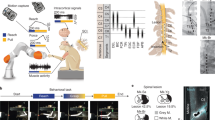
Abstract
Patients with spinal cord injury lack the connections between brain and spinal cord circuits that are essential for voluntary movement. Clinical systems that achieve muscle contraction through functional electrical stimulation (FES) have proven to be effective in allowing patients with tetraplegia to regain control of hand movements and to achieve a greater measure of independence in daily activities1,2. In existing clinical systems, the patient uses residual proximal limb movements to trigger pre-programmed stimulation that causes the paralysed muscles to contract, allowing use of one or two basic grasps. Instead, we have developed an FES system in primates that is controlled by recordings made from microelectrodes permanently implanted in the brain. We simulated some of the effects of the paralysis caused by C5 or C6 spinal cord injury3 by injecting rhesus monkeys with a local anaesthetic to block the median and ulnar nerves at the elbow. Then, using recordings from approximately 100 neurons in the motor cortex, we predicted the intended activity of several of the paralysed muscles, and used these predictions to control the intensity of stimulation of the same muscles. This process essentially bypassed the spinal cord, restoring to the monkeys voluntary control of their paralysed muscles. This achievement is a major advance towards similar restoration of hand function in human patients through brain-controlled FES. We anticipate that in human patients, this neuroprosthesis would allow much more flexible and dexterous use of the hand than is possible with existing FES systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Keith, M. W. et al. Implantable functional neuromuscular stimulation in the tetraplegic hand. J. Hand Surg. Am. 14, 524–530 (1989)
Peckham, P. H. et al. Efficacy of an implanted neuroprosthesis for restoring hand grasp in tetraplegia: a multicenter study. Arch. Phys. Med. Rehabil. 82, 1380–1388 (2001)
Pohlmeyer, E. A., Jordon, L. R., Kim, P. & Miller, L. E. A fully implanted drug delivery system for peripheral nerve blocks in behaving animals. J. Neurosci. Methods 182, 165–172 (2009)
International. Campaign for Cures of Spinal Cord Injury Paralysis. http://www.campaignforcure.org (2011)
Anderson, K. D. Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma 21, 1371–1383 (2004)
Popovic, M. R., Popovic, D. B. & Keller, T. Neuroprostheses for grasping. Neurol. Res. 24, 443–452 (2002)
Kilgore, K. L. et al. An implanted upper-extremity neuroprosthesis. Follow-up of five patients. J. Bone Joint Surg. Am. 79, 533–541 (1997)
Evarts, E. V. Relation of pyramidal tract activity to force exerted during voluntary movement. J. Neurophysiol. 31, 14–27 (1968)
Humphrey, D. R., Schmidt, E. M. & Thompson, W. D. Predicting measures of motor performance from multiple cortical spike trains. Science 170, 758–761 (1970)
Carmena, J. M. et al. Learning to control a brain–machine interface for reaching and grasping by primates. PLoS Biol. 1, e42 (2003)
Gupta, R. & Ashe, J. Offline decoding of end-point forces using neural ensembles: application to a brain–machine interface. Neural Systems and Rehabilitation Engineering. IEEE Trans. Neural Syst. Rehabil. Eng. 17, 254–262 (2009)
Fagg, A. H., Ojakangas, G. W., Miller, L. E. & Hatsopoulos, N. G. Kinetic trajectory decoding using motor cortical ensembles. IEEE Trans. Neural Syst. Rehabil. Eng. 17, 487–496 (2009)
Pohlmeyer, E. A., Solla, S. A., Perreault, E. J. & Miller, L. E. Prediction of upper limb muscle activity from motor cortical discharge during reaching. J. Neural Eng. 4, 369–379 (2007)
Cherian, A., Krucoff, M. O. & Miller, L. E. Motor cortical prediction of EMG: evidence that a kinetic brain-machine interface may be robust across altered movement dynamics. J. Neurophysiol. 106, 564–575 (2011)
Pohlmeyer, E. A. et al. Real-time control of the hand by intracortically controlled functional neuromuscular stimulation. IEEE 10th Int. Conf. Rehab. Robotics 454–458 (2007)
Pohlmeyer, E. A. et al. Toward the restoration of hand use to a paralyzed monkey: Brain-controlled functional electrical stimulation of forearm muscles. PLoS ONE 4, e5924 (2009)
Oby, E. R. et al. in Statistical Signal Processing for Neuroscience and Neurotechnology (ed. O’Weiss, K.G. ) 369–406 (Academic Press, 2010)
Moritz, C. T., Perlmutter, S. I. & Fetz, E. E. Direct control of paralysed muscles by cortical neurons. Nature 456, 639–642 (2008)
Adams, M. M. & Hicks, A. L. Spasticity after spinal cord injury. Spinal Cord 43, 577–586 (2005)
Kern, H. et al. Denervated muscles in humans: limitations and problems of currently used functional electrical stimulation training protocols. Artif. Organs 26, 216–218 (2002)
Waters, R. L., Adkins, R. H., Yakura, J. S. & Sie, I. Motor and sensory recovery following complete tetraplegia. Arch. Phys. Med. Rehabil. 74, 242–247 (1993)
Bryden, A. M. et al. An implanted neuroprosthesis for high tetraplegia. Top. Spinal Cord Inj. Rehabil. 10, 38–52 (2005)
Nightingale, E. J., Raymond, J., Middleton, J. W., Crosbie, J. & Davis, G. M. Benefits of FES gait in a spinal cord injured population. Spinal Cord 45, 646–657 (2007)
Agarwal, S. et al. Long-term user perceptions of an implanted neuroprosthesis for exercise, standing, and transfers after spinal cord injury. J. Rehabil. Res. Dev. 40, 241–252 (2003)
Fitzwater, R. A personal user’s view of functional electrical stimulation cycling. Artif. Organs 26, 284–286 (2002)
Acknowledgements
This work was supported in part by grant NS053603 from the National Institute of Neurological Disorders and Stroke to L.E.M. and a post-doctoral fellowship from the Fonds de la Recherche en Santé du Québec to C.E., with further support from the Chicago Community Trust through the Searle Program for Neurological Restoration at the Rehabilitation Institute of Chicago. We also acknowledge the technical assistance of D. Tyler and K. Kilgore as well as the surgical assistance of J. Ko and S. Paisley Agnew.
Author information
Authors and Affiliations
Contributions
L.E.M. conceived, designed and supervised the basic experiments. C.E. and E.R.O. performed the experiments. M.J.B. carried out software development. C.E. analysed the data and prepared figures. L.E.M. and C.E. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Methods and Results, and Supplementary Figures 1-5. (PDF 418 kb)
Supplementary Movie 1
This movie shoes several examples of Monkey T executing the ball grasp experiment during FES and catch trials. (MOV 3319 kb)
Supplementary Movie 2
This movie shows several examples of Monkey J executing the ball grasp experiment during normal, FES and catch trials. (MOV 3899 kb)
Rights and permissions
About this article
Cite this article
Ethier, C., Oby, E., Bauman, M. et al. Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature 485, 368–371 (2012). https://doi.org/10.1038/nature10987
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10987
This article is cited by
-
Brain control of bimanual movement enabled by recurrent neural networks
Scientific Reports (2024)
-
Neurotechnologies to restore hand functions
Nature Reviews Bioengineering (2023)
-
An implantable, wireless, battery-free system for tactile pressure sensing
Microsystems & Nanoengineering (2023)
-
Implantable intracortical microelectrodes: reviewing the present with a focus on the future
Microsystems & Nanoengineering (2023)
-
Intracortical brain-computer interfaces in primates: a review and outlook
Biomedical Engineering Letters (2023)