Abstract
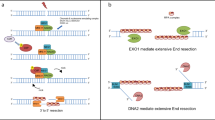
Repair of DNA double-strand breaks (DSBs) by homologous recombination requires resection of 5′-termini to generate 3′-single-strand DNA tails1. Key components of this reaction are exonuclease 1 and the bifunctional endo/exonuclease, Mre11 (refs 2–4). Mre11 endonuclease activity is critical when DSB termini are blocked by bound protein—such as by the DNA end-joining complex5, topoisomerases6 or the meiotic transesterase Spo11 (refs 7–13)—but a specific function for the Mre11 3′–5′ exonuclease activity has remained elusive. Here we use Saccharomyces cerevisiae to reveal a role for the Mre11 exonuclease during the resection of Spo11-linked 5′-DNA termini in vivo. We show that the residual resection observed in Exo1-mutant cells is dependent on Mre11, and that both exonuclease activities are required for efficient DSB repair. Previous work has indicated that resection traverses unidirectionally1. Using a combination of physical assays for 5′-end processing, our results indicate an alternative mechanism involving bidirectional resection. First, Mre11 nicks the strand to be resected up to 300 nucleotides from the 5′-terminus of the DSB—much further away than previously assumed. Second, this nick enables resection in a bidirectional manner, using Exo1 in the 5′–3′ direction away from the DSB, and Mre11 in the 3′–5′ direction towards the DSB end. Mre11 exonuclease activity also confers resistance to DNA damage in cycling cells, suggesting that Mre11-catalysed resection may be a general feature of various DNA repair pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Paques, F. & Haber, J. E. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63, 349 (1999)
Mimitou, E. P. & Symington, L. S. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature 455, 770–774 (2008)
Nicolette, M. L. et al. Mre11–Rad50–Xrs2 and Sae2 promote 5′ strand resection of DNA double-strand breaks. Nature Struct. Mol. Biol. 17, 1478–1485 (2010)
Zhu, Z., Chung, W. H., Shim, E. Y., Lee, S. E. & Ira, G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell 134, 981–994 (2008)
Mimitou, E. P. & Symington, L. S. Ku prevents Exo1 and Sgs1-dependent resection of DNA ends in the absence of a functional MRX complex or Sae2. EMBO J. 29, 3358–3369 (2010)
Hartsuiker, E., Neale, M. J. & Carr, A. M. Distinct requirements for the Rad32(Mre11) nuclease and Ctp1(CtIP) in the removal of covalently bound topoisomerase I and II from DNA. Mol. Cell 33, 117–123 (2009)
Furuse, M. et al. Distinct roles of two separable in vitro activities of yeast Mre11 in mitotic and meiotic recombination. EMBO J. 17, 6412–6425 (1998)
Hartsuiker, E. et al. Ctp1CtIP and Rad32Mre11 nuclease activity are required for Rec12Spo11 removal, but Rec12Spo11 removal is dispensable for other MRN-dependent meiotic functions. Mol. Cell. Biol. 29, 1671–1681 (2009)
Milman, N., Higuchi, E. & Smith, G. R. Meiotic DNA double-strand break repair requires two nucleases, MRN and Ctp1, to produce a single size class of Rec12 (Spo11)-oligonucleotide complexes. Mol. Cell. Biol. 29, 5998–6005 (2009)
Moreau, S., Ferguson, J. R. & Symington, L. S. The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol. Cell. Biol. 19, 556–566 (1999)
Nairz, K. & Klein, F. mre11S—a yeast mutation that blocks double-strand-break processing and permits nonhomologous synapsis in meiosis. Genes Dev. 11, 2272–2290 (1997)
Rothenberg, M., Kohli, J. & Ludin, K. Ctp1 and the MRN-complex are required for endonucleolytic Rec12 removal with release of a single class of oligonucleotides in fission yeast. PLoS Genet. 5, e1000722 (2009)
Tsubouchi, H. & Ogawa, H. A novel mre11 mutation impairs processing of double-strand breaks of DNA during both mitosis and meiosis. Mol. Cell. Biol. 18, 260–268 (1998)
Keeney, S. Mechanism and control of meiotic recombination initiation. Curr. Top. Dev. Biol. 52, 1–53 (2001)
Neale, M. J., Pan, J. & Keeney, S. Endonucleolytic processing of covalent protein-linked DNA double-strand breaks. Nature 436, 1053–1057 (2005)
Williams, R. S. et al. Mre11 dimers coordinate DNA end bridging and nuclease processing in double-strand-break repair. Cell 135, 97–109 (2008)
Hodgson, A. et al. Mre11 and Exo1 contribute to the initiation and processivity of resection at meiotic double-strand breaks made independently of Spo11. DNA Repair 10, 138–148 (2010)
Keelagher, R. E., Cotton, V. E., Goldman, A. S. & Borts, R. H. Separable roles for Exonuclease I in meiotic DNA double-strand break repair. DNA Repair 10, 126–137 (2010)
Manfrini, N., Guerini, I., Citterio, A., Lucchini, G. & Longhese, M. P. Processing of meiotic DNA double-strand breaks requires cyclin-dependent kinase and multiple nucleases. J. Biol. Chem. 285, 11628–11637 (2010)
Tsubouchi, H. & Ogawa, H. Exo1 roles for repair of DNA double-strand breaks and meiotic crossing over in Saccharomyces cerevisiae. Mol. Biol. Cell 11, 2221–2233 (2000)
Zakharyevich, K. et al. Temporally and biochemically distinct activities of Exo1 during meiosis: double-strand break resection and resolution of double holliday junctions. Mol. Cell 40, 1001–1015 (2010)
Carballo, J. A., Johnson, A. L., Sedgwick, S. G. & Cha, R. S. Phosphorylation of the axial element protein Hop1 by Mec1/Tel1 ensures meiotic interhomolog recombination. Cell 132, 758–770 (2008)
Usui, T., Ogawa, H. & Petrini, J. H. A. DNA damage response pathway controlled by Tel1 and the Mre11 complex. Mol. Cell 7, 1255–1266 (2001)
Szankasi, P. & Smith, G. R. A. DNA exonuclease induced during meiosis of Schizosaccharomyces pombe. J. Biol. Chem. 267, 3014–3023 (1992)
Paull, T. T. & Gellert, M. The 3′ to 5′ exonuclease activity of Mre11 facilitates repair of DNA double-strand breaks. Mol. Cell 1, 969–979 (1998)
Pan, J. et al. A hierarchical combination of factors shapes the genome-wide topography of yeast meiotic recombination initiation. Cell 144, 719–731 (2011)
Jazayeri, A., Balestrini, A., Garner, E., Haber, J. E. & Costanzo, V. Mre11–Rad50–Nbs1-dependent processing of DNA breaks generates oligonucleotides that stimulate ATM activity. EMBO J. 27, 1953–1962 (2008)
Lange, J. et al. ATM controls meiotic double-strand-break formation. Nature 10.1038/nature10508 (this issue).
Hunter, N. & Kleckner, N. The single-end invasion: an asymmetric intermediate at the double-strand break to double-holliday junction transition of meiotic recombination. Cell 106, 59–70 (2001)
Kim, K. P. et al. Sister cohesion and structural axis components mediate homolog bias of meiotic recombination. Cell 143, 924–937 (2010)
Panizza, S. et al. Spo11-accessory proteins link double-strand break sites to the chromosome axis in early meiotic recombination. Cell 146, 372–383 (2011)
Acknowledgements
We thank R. Cha, E. Hoffmann, N. Hunter, S. Keeney and J. Nitiss for yeast strains; L. Symington for plasmids; F. Klein and J. Petrini for antisera. V.G. is supported by an MRC New Investigator Grant to M.J.N. M.J.N. is supported by a University Research Fellowship from the Royal Society and a Career Development Award from the Human Frontiers Science Program Organisation.
Author information
Authors and Affiliations
Contributions
V.G. and M.J.N. designed the experiments and wrote the paper. V.G. and M.J.N. performed the experiments with technical support from S.E.L.P. and S.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-10 with legends and additional references. (PDF 5963 kb)
Rights and permissions
About this article
Cite this article
Garcia, V., Phelps, S., Gray, S. et al. Bidirectional resection of DNA double-strand breaks by Mre11 and Exo1. Nature 479, 241–244 (2011). https://doi.org/10.1038/nature10515
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10515