Abstract
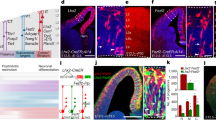
Detailed analysis of neuronal network architecture requires the development of new methods. Here we present strategies to visualize synaptic circuits by genetically labelling neurons with multiple, distinct colours. In Brainbow transgenes, Cre/lox recombination is used to create a stochastic choice of expression between three or more fluorescent proteins (XFPs). Integration of tandem Brainbow copies in transgenic mice yielded combinatorial XFP expression, and thus many colours, thereby providing a way to distinguish adjacent neurons and visualize other cellular interactions. As a demonstration, we reconstructed hundreds of neighbouring axons and multiple synaptic contacts in one small volume of a cerebellar lobe exhibiting approximately 90 colours. The expression in some lines also allowed us to map glial territories and follow glial cells and neurons over time in vivo. The ability of the Brainbow system to label uniquely many individual cells within a population may facilitate the analysis of neuronal circuitry on a large scale.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Denk, W. & Horstmann, H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2, e329 (2004)
Briggman, K. L. & Denk, W. Towards neural circuit reconstruction with volume electron microscopy techniques. Curr. Opin. Neurobiol. 16, 562–570 (2006)
Micheva, K. D. & Smith, S. J. Array tomography: a new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron 55, 25–36 (2007)
Gan, W. B., Grutzendler, J., Wong, W. T., Wong, R. O. & Lichtman, J. W. Multicolor “DiOlistic” labeling of the nervous system using lipophilic dye combinations. Neuron 27, 219–225 (2000)
Shaner, N. C., Steinbach, P. A. & Tsien, R. Y. A guide to choosing fluorescent proteins. Nature Methods 2, 905–909 (2005)
Hadjantonakis, A. K., Macmaster, S. & Nagy, A. Embryonic stem cells and mice expressing different GFP variants for multiple non-invasive reporter usage within a single animal. BMC Biotechnol. 2, 11 (2002)
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000)
Kasthuri, N. & Lichtman, J. W. The role of neuronal identity in synaptic competition. Nature 424, 426–430 (2003)
Zong, H., Espinosa, J. S., Su, H. H., Muzumdar, M. D. & Luo, L. Mosaic analysis with double markers in mice. Cell 121, 479–492 (2005)
Branda, C. S. & Dymecki, S. M. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev. Cell 6, 7–28 (2004)
Lam, K. P. & Rajewsky, K. Rapid elimination of mature autoreactive B cells demonstrated by Cre-induced change in B cell antigen receptor specificity in vivo . Proc. Natl Acad. Sci. USA 95, 13171–13175 (1998)
Lee, G. & Saito, I. Role of nucleotide sequences of loxP spacer region in Cre-mediated recombination. Gene 216, 55–65 (1998)
Caroni, P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J. Neurosci. Methods 71, 3–9 (1997)
Guo, C., Yang, W. & Lobe, C. G. A Cre recombinase transgene with mosaic, widespread tamoxifen-inducible action. Genesis 32, 8–18 (2002)
Metzger, D., Clifford, J., Chiba, H. & Chambon, P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc. Natl Acad. Sci. USA 92, 6991–6995 (1995)
Garrick, D., Fiering, S., Martin, D. I. & Whitelaw, E. Repeat-induced gene silencing in mammals. Nature Genet. 18, 56–59 (1998)
Palmiter, R. D. & Brinster, R. L. Germ-line transformation of mice. Annu. Rev. Genet. 20, 465–499 (1986)
Rodriguez, C. I. & Dymecki, S. M. Origin of the precerebellar system. Neuron 27, 475–486 (2000)
Rowan, S. & Cepko, C. L. Genetic analysis of the homeodomain transcription factor Chx10 in the retina using a novel multifunctional BAC transgenic mouse reporter. Dev. Biol. 271, 388–402 (2004)
Eccles, J. C., Ito, M. & Szentágothai, J. in The Cerebellum as a Neuronal Machine (Springer, Berlin, 1967)
Wu, H. S., Sugihara, I. & Shinoda, Y. Projection patterns of single mossy fibers originating from the lateral reticular nucleus in the rat cerebellar cortex and nuclei. J. Comp. Neurol. 411, 97–118 (1999)
Palay, S. L. & Chan-Palay, V. in Cerebellar Cortex (Springer, New York, 1974)
Bushong, E. A., Martone, M. E., Jones, Y. Z. & Ellisman, M. H. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 22, 183–192 (2002)
Ogata, K. & Kosaka, T. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience 113, 221–233 (2002)
Silver, D. P. & Livingston, D. M. Self-excising retroviral vectors encoding the Cre recombinase overcome Cre-mediated cellular toxicity. Mol. Cell 8, 233–243 (2001)
Gong, S. et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925 (2003)
Lein, E. S. et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007)
Zacharias, D. A., Violin, J. D., Newton, A. C. & Tsien, R. Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296, 913–916 (2002)
Rizzo, M. A., Springer, G. H., Granada, B. & Piston, D. W. An improved cyan fluorescent protein variant useful for FRET. Nature Biotechnol. 22, 445–449 (2004)
Karasawa, S., Araki, T., Nagai, T., Mizuno, H. & Miyawaki, A. Cyan-emitting and orange-emitting fluorescent proteins as a donor/acceptor pair for fluorescence resonance energy transfer. Biochem. J. 381, 307–312 (2004)
Campbell, R. E. et al. A monomeric red fluorescent protein. Proc. Natl Acad. Sci. USA 99, 7877–7882 (2002)
Shaner, N. C. et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nature Biotechnol. 22, 1567–1572 (2004)
Kay, J. N. et al. Transient requirement for ganglion cells during assembly of retinal synaptic layers. Development 131, 1331–1342 (2004)
Buffelli, M. et al. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature 424, 430–434 (2003)
Zuo, Y. et al. Fluorescent proteins expressed in mouse transgenic lines mark subsets of glia, neurons, macrophages, and dendritic cells for vital examination. J. Neurosci. 24, 10999–11009 (2004)
Fiala, J. C. Reconstruct: a free editor for serial section microscopy. J. Microsc. 218, 52–61 (2005)
Kakoki, M. et al. Altering the expression in mice of genes by modifying their 3′ regions. Dev. Cell 6, 597–606 (2004)
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001)
Marquardt, T. et al. Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105, 43–55 (2001)
Gorski, J. A. et al. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 22, 6309–6314 (2002)
Ringrose, L., Chabanis, S., Angrand, P. O., Woodroofe, C. & Stewart, A. F. Quantitative comparison of DNA looping in vitro and in vivo: chromatin increases effective DNA flexibility at short distances. EMBO J. 18, 6630–6641 (1999)
Zheng, B., Sage, M., Sheppeard, E. A., Jurecic, V. & Bradley, A. Engineering mouse chromosomes with Cre-loxP: range, efficiency, and somatic applications. Mol. Cell. Biol. 20, 648–655 (2000)
Acknowledgements
We thank members of the Lichtman and Sanes laboratories for discussions; H. Nishimune for insights; A. Mendelsohn and A. Bruskin for cell culture and PCR; S.-H. Shieu and D. Mou for imaging software development; S. Haddad, D. Pelusi, D. Malkowski, K. Mahoney and the Harvard MCB BRI facility for animal care and genotyping; M. Wallace, the WUSTL Mouse Genetic Core and the MCB Genome Manipulation Facility for pronuclei injections; and R. Hellmiss for help with graphics. We thank J. C. Fiala for assistance with Reconstruct; M. L. Nonet, J. S. Mumm and R. O. Wong for advice and reagents; D. W. Piston for mCerulean; R. Y. Tsien for tdimer2 and mCherry; and A. Miyawaki for Kusabira. This work was supported by the James S. McDonnell foundation and grants from NIH.
Author Contributions J.L., J.R.S. and J.W.L. conceived the Brainbow strategies. J.R.S. and J.W.L. supervised the project. J.L. built initial constructs and validated them in vitro and in vivo. T.A.W. performed all cerebellar axonal tracing and colour profile analysis with programs developed with J. Lu. H.K. performed all live imaging experiments. R.W.D. generated Brainbow-1.0 lines expressing cytoplasmic XFPs, and R.A.B. generated Brainbow-1.1 constructs and lines. J.L., T.A.W. and R.W.D. screened mouse lines.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-6 with Legends and Supplementary Tables 1-2. (PDF 10125 kb)
Supplementary Movie 1
The file contains Supplementary Movie 1 which shows rotation of the cerebellar reconstruction. (MOV 39667 kb)
Rights and permissions
About this article
Cite this article
Livet, J., Weissman, T., Kang, H. et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 (2007). https://doi.org/10.1038/nature06293
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature06293
This article is cited by
-
Whole-body cellular mapping in mouse using standard IgG antibodies
Nature Biotechnology (2024)
-
Clonal tracking in cancer and metastasis
Cancer and Metastasis Reviews (2024)
-
Cell cycle dynamics control fluidity of the developing mouse neuroepithelium
Nature Physics (2023)
-
Inflationary theory of branching morphogenesis in the mouse salivary gland
Nature Communications (2023)
-
Brain imaging turned inside out
Nature Biotechnology (2023)