Abstract
We present an uncommon case (female patient aged 59 years) of the clear-cell variant of calcifying epithelial odontogenic tumor (CEOT) (also known as Pindborg tumor) in the mandible. The clinical characteristics and probable origins of the clear tumor cells of previously reported cases of clear-cell variant of intraosseous CEOT are also summarized and discussed.
Similar content being viewed by others
Introduction
Calcifying epithelial odontogenic tumor (CEOT) (also known as Pindborg tumor), which was first designated as a distinct disease entity by Pindborg, is an uncommon benign odontogenic lesion that accounts for less than 1% of all odontogenic tumors. It most often occurs in the posterior mandible and is most frequently found in patients between 30 and 50 years of age, with no sex predilection.1 In addition to the intraosseous lesion, a number of extraosseous counterparts of CCEOT have also been documented.1 Clinically, CEOT is usually a slow-growing painless swelling. Radiographically, a unilocular radiolucency destructive lesion is observed. The classical histopathological characteristics of CEOT comprise sheets and islands of polyhedral eosinophilic epithelial cells with calcifications as well as deposition of an amyloid-like substance; however, occasionally, focal areas of clear cells can be observed in the clear-cell variant of CEOT (CCCEOT).2
Through a MEDLINE search for CCCEOT in the English-language literature (1967–2011), 14 cases were found;3,4,5,6,7,8,9,10,11,12,13,14,15,16 however, this unusual lesion still needs continual documentation in order to have more information regarding clinical, microscopic features or behavior, particularly, the potential origins of the clear tumor cells. Therefore, the aim of the current report was to describe the clinical, radiographic, and histological findings in a case of mandibular CCCEOT. The clinical features as well as the potential origins of the clear tumor cells of previously reported cases of intraosseous CCCEOT are reviewed.
Case report
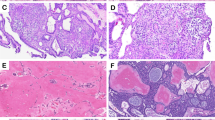
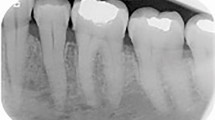
A 59-year-old female was referred for evaluation of a painless swelling over the left retromolar area. The patient’s medical history was significant for the diagnosis of hypertension. Intraoral examination showed a hard, non-tender 3 cm×2 cm mass on the lingual aspect of the left retromolar area up to half of the mandibular ramus. The overlying mucosa was intact (Figure 1a). A panoramic radiograph showed a well-defined unilocular radiolucence with a corticated margin extending from the distal root of tooth 38 up to half of the left ramus area, and from the left retromolar area down to the mandibular body, which measured about 3 cm×2 cm in diameter (Figure 1b). The differential diagnosis included keratocystic odontogenic tumor, ameloblastoma, ameloblastic fibroma and CEOT. An incisional biopsy was performed under local anesthesia. The specimen was sent to the Oral Pathology Department of our institution for histological examination. Microscopic examination of the incisional biopsy showed that a large portion of the tumor was arranged in a pseudoglandular pattern consisting of nests of pale, uniform, clear cells with dark-stained nuclei without abnormal mitotic figures and necrosis (Figure 2a), whereas some areas were admixed with clusters of polyhedral epithelial cells (Figure 2b). The cells were separated by thin bands of connective tissue in areas showing deposits of amorphous eosinophilic material. Small foci of calcifications were also noted, but no Liesegang rings were observed (Figure 2c). Staining was negative for periodic acid fast stain (PAS) stains with and without diastase digestion (data not shown), as well as mucicarmine stain (data not shown), but positive for Congo red stain throughout the intercellular eosinophilic material (Figure 3a). With regard to immunohistochemical stainings, the tumor cells were positive for cytokeratin only (Figure 3b), and negative for S-100 protein (Figure 3c) and smooth muscle actin (Figure 3d). The findings for Ki-67 were positive in only a small number of scattered cells (Figure 3e). Therefore, the histological diagnosis was CCCEOT.
Intraoral view and panoramic radiography. (a) Intraoral examination showed a mass on the left retromolar area up to half of the mandibular ramus. (b) Panoramic radiograph showed a well-defined unilocular radiolucence with a corticated margin extending from the distal root of tooth 38 up to half of the left ramus area, and from the left retromolar area down to the mandibular body.
Histological aspects of the incisional and excisional biopsies. Incisional biopsy showed that a large portion of the tumor was arranged in a pseudoglandular pattern consisting of nests of pale, uniform, clear cells with dark-stained nuclei (a, ×40), whereas some areas were admixed with polyhedral epithelial cells (b, ×100) and contained small foci of calcification (arrow, c, ×200). (d) Similar histopathological findings to the incisional biopsy were observed for the surgical specimen (×40).
Histochemical and immunohistochemical aspects. Staining was positive for Congo red stain for the intercellular eosinophilic material (a, ×200). The tumor cells were positive for immunochemical staining of cytokeratin (b, ×100), negative for S-100 (c, ×100) and smooth muscle actin (d, ×200) as well as low Ki-67 labeling index (e, ×100).
The swelling was then removed under general anesthesia. Similar microscopic findings to the incisional biopsy were observed for the surgical specimen (Figure 2d). The histological diagnosis of the surgical specimen was confirmed again to be CCCEOT. The postoperative course of the patient was uneventful, and there was no evidence of disease at the 2-year follow-up.
Discussion
The histopathology of CEOT, in its classic pattern, comprises sheets of polyhedral epithelial cells with well-defined cell borders and distinct intercellular bridges; these neoplastic cells may demonstrate pleomorphism, but only rarely typical mitoses. Additionally, the other most characteristic findings are the presence of amyloid-like substances and calcified concentric Liesegang rings. To date, five histopathologic patterns of CEOT have been documented:17,18 (i) strands/sheets/islands of polyhedral cells with intracellular bridges; (ii) a cribriform arrangement with many spaces containing an eosinophilic (amyloid-like) substance; (iii) densely-populated neoplastic cells with interspersed multinucleated giant cells; (iv) nests of epithelial cells similar to neoplasm of the salivary gland; and (v) prominent clear-cell arranged in a pseudoglandular manner. The last pattern is referred to as the clear-cell variant of CEOT, and the histopathological findings of the current case were consistent with this pattern, showing abundant clear cells arranged in a pseudoglandular pattern containing an amyloid-like material.
In the current case, a relatively high proportion of the clear tumor cell components were observed. The diagnosis of CCCEOT in the present case was reached according to the positivity of cytokeratin staining, the absence of PAS-positive staining, the presence of Congo red-positive material between tumor islands (amyloid-like material) and the absence of mitotic figures. Malignance of salivary gland origin was ruled out by the absence of actin and S-100 expression, and clear-cell odontogenic carcinoma was ruled out by the lack of overt cellular atypia, a well-circumscribed lesion, and the presence of amyloid-like material as well as the very low Ki-67 labeling index. Additionally, a lack of mitotic figures and the generally good circumscription of the lesion are not characteristics of metastatic diseases of any origin.
It should be noted that clear cells may also occur in other epithelial odontogenic leisons such as ameloblastoma,19 and calcifying odontogenic cyst.20 It has been demonstrated that the clear cells of the ameloblastoma was clearly of odontogenic epithelial origin.19 Moreover, it has also been of opinion that the clear cells of the calcifying odontogenic cyst are possibly odontogenic epithelial cells, which have undergone aberrant degeneration.20
For CCCEOT, it has been claimed that the clear cells represent a degenerative process,4,10 whereas another suggestion indicated that the clear tumor cells represent a feature of cytodifferentiation rather than the degenerative phenomenon.8 In order to obtain some valuable information, we hereby summarize the reported histochemical, immnohistochemical, and electron microscopic findings (including the current case) for the potential origins of clear tumor cells in CCCEOT in Table 1.
As observed from Table 1, in two cases, the clear tumor cells have been proved to be Langerhans cells, as evidenced by the presence of Birbeck’s granules using electron microscopy.9,14 Indeed, some authors have regarded CEOT containing Langerhans cells as non-calcifying CEOT (Pindborg tumor) with Langerhans cells. On the other hand, it has been shown that the clear cells of CCCEOT contain glycogen in four cases by PAS stain8,12,13,15 and in two cases by electron microscopy.8,13 An odontogenic epithelial origin has also been demonstrated for the clear tumor cells in three cases by positive immunohistochemical stainings (chiefly cytokeratin) together with negative stainings for PAS and mucicarmine stains13,14 and the present case, and in one case by electron microscopy.10 Both PAS and cytokeratin positivity have been simultaneously reported in one case, in which transition between clear cells and CEOT tumor cells was evident throughout the tumor tissues.13 Taken together, it may be speculated that the clear-cell change might be derived from the cytokeratin-positive CEOT tumor cells; these tumor cells initially showed a congregation of initially PAS-positive substance, but when the clear-cell phenomenon enhances, the PAS positivity is consistently absent.10 Consequently, the potential origin and function of the clear tumor cells in CCCEOT would be more complex as compared to other epithelial odontogenic lesions,19,20 and the clear-cells in CCCEOT may in fact, represent a more diversity of cell origins encompassing Langerhans cells, degenerated cells containing glycogen granules, and cells of overt odontogenic epithelial origin.
Additionally, one may also question whether the presence of clear cells is of clinical relevance. In 1994, Hicks et al.12 suggested that the existence of clear tumor cells in CCCEOT may imply a more aggressive performance. However, other authors considered that too few cases of CCCEOT have been described to date to attain a confirmative conclusion concerning the impact of the clear-cell population on the biologic activity of CCCEOT (ref. 2). Today, the choice of surgical management of the CEOT depends on the site, size, and amount of bone destruction of the lesion. For mandiblular lesions, the suggested surgical approach is enucleation with vigorous curettage; however, for lesions with more advanced bone infiltration, resection of the tumor should be considered.1,2 The treatment of choice in the current mandibular case is total excision of the tumor. On the other hand, hemimaxillectomy is suggested as the treatment of choice for lesions of the maxilla, because maxilla tumors could easily intrude on vital structures.1,2
Finally, Anavi et al.14 presented a clinical review of CCCEOT in 2003. After almost ten years, as shown in Table 1, we have updated the data of Anavi et al.14 by adding three more cases,15,16 including the present case. With only three additional cases, it is unsurprising to observe that the main clinical data shown in Table 1 are largely compatible with the report of Anavi et al.14
Conclusions
We report an uncommon case of CCCEOT arising in the mandible, and additionally, a review of pertinent literature as well as discussion of the potential origins of the clear tumor cells have been presented.
References
Neville B, Damm DD, Allen CM et al. Oral and Maxillofacial Pathology. 3rd ed. Philadelphia: WB Saunders, 2009: 716–718.
Philipsen HP, Reichart PA . Calcifying epithelial odontogenic tumor: biological profile based on 181 cases from the literature. Oral Oncol 2000; 36( 1): 17–26.
Abrams AM, Howell FV . Calcifying epithelial odontogenic tumors: report of four cases. J Am Dent Assoc 1967; 74( 6): 1231–1240.
Anderson HC, Kim B, Minkowitz S . Calcifying epithelial odontogenic tumor of Pindborg: an electron microscopic study. Cancer 1969; 24( 3): 585–596.
Greer RO, Richardson JF . Clear-cell calcifying odontogenic tumor viewed relative to the Pindborg tumor. Oral Surg Oral Med Oral Pathol 1971; 42( 6): 775–779.
Wallace J, MacDonald GD . Calcifying epithelial odontogenic tumour (“Pindborg tumour”): a case report. Br J Plast Surg 1974; 27( 1): 28–30.
Oikarinen VJ, Calonius PEB, Meretoja J . Calcifying epithelial odontogenic tumor (Pindborg tumor): case report. Int J Oral Surg 1976; 5( 4): 187–191.
Yamaguchi A, Kokubu JM, Takagi M et al. Calcifying odontogenic tumor: histochemical and electron microscopic observation of a case. Bull Tokyo Med Dent Univ 1980; 27( 3): 129–135.
Asano M, Takahashi T, Kusama K et al. A variant of calcifying epithelial odontogenic tumor with Langerhans cells. J Oral Pathol Med 1990; 19( 9): 430–434.
Schmidt-Westhausen A, Philipsen HP, Reichart PA . Clear cell calcifying epithelial odontogenic tumor: a case report. Int J Oral Maxillofac Surg 1992; 21( 1): 47–49.
Takata T, Ogawa I, Miyauchi M et al. Non-calcifying Pindborg tumor with Langerhans cells. J Oral Pathol Med 1993; 22( 8): 378–383.
Hicks MJ, Flaitz CM, Wong ME et al. Clear cell variant of calcifying epithelial odontogenic tumor: case report and review of the literature. Head Neck 1994; 16( 3): 272–277.
Kumamoto H, Sato I, Tateno H et al. Clear cell variant of calcifying epithelial odontogenic tumor (CEOT) in the maxilla: report of a case with immunohistochemical and ultrastructural investigations. J Oral Pathol Med 1999; 28( 4): 187–191.
Anavi Y, Kaplan I, Citir M et al. Clear-cell variant of calcifying epithelial odontogenic tumor: clinical and radiographic characteristics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 95( 3): 332–339.
Germanier Y, Bornstein MM, Stauffer E et al. Calcifying epithelial odontogenic (Pindborg) tumor of the mandible with clear cell component treated by conservative surgery: report of a case. J Oral Maxillofac Surg 2005; 63( 9): 1377–1382.
Mohtasham N, Habibi A, Jafarzadeh H et al. Extension of Pindborg tumor to the maxillary sinus: a case report. J Oral Pathol Med 2008; 37( 1): 59–61.
Krolls SO, Pindborg JJ . Calcifying epithelial odontogenic tumor. A survey of 23 cases and discussion of histomorphologic variations. Arch Pathol 1974; 98( 3): 206–210.
Pindborg JJ, Vedtofte P, Reibel J et al. The calcifying epithelial odontogenic tumor. A review of recent literature and report of a case. APMIS 1991; 23( Suppl): 152–157.
de Aguiar MC, Gomez RS, Silva EC et al. Clear-cell ameloblastoma (clear-cell odontogenic carcinoma): report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996; 81( 1): 79–83.
Ng KH, Siar CH . Clear cell change in a calcifying odontogenic cyst. Oral Surg Oral Med Oral Pathol 1985; 60( 4): 417–419.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Chen, CY., Wu, CW., Wang, WC. et al. Clear-cell variant of calcifying epithelial odontogenic tumor (Pindborg tumor) in the mandible. Int J Oral Sci 5, 115–119 (2013). https://doi.org/10.1038/ijos.2013.29
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijos.2013.29
Keywords
This article is cited by
-
Non-Calcifying/Langerhans Cell-Rich Calcifying Epithelial Odontogenic Tumour: A Critical Review of the Rare and Distinctive Entity
Head and Neck Pathology (2023)
-
CEOT Variants or Entities: Time for a Rethink? A Case Series with Review of the Literature
Head and Neck Pathology (2021)