Abstract
Recent studies have indicated that periodontopathic bacteria might accelerate the development of cardiac fibrosis. Porphyromonas gingivalis (P. gingivalis), a major periodontal bacterium, is mainly recognized by Toll-like receptor-2 (TLR-2). However, the role of TLR-2 in the acceleration of cardiac fibrosis via infections caused by periodontal bacteria has not yet been investigated. Here we investigated the role TLR-2 has in periodontal pathogen-induced cardiac fibrosis. TLR-2 knockout (KO) and wild type (WT) male C57BL/6 mice were subjected to a transverse aortic constriction (TAC) surgical procedure 2 weeks after chamber implantation. After the TAC operation, mice received injections once a week of P. gingivalis or vehicle into the chambers that were implanted in the back of mice. Fractional shortening (FS) was measured using echocardiography 1 week after the TAC surgical procedure. Four weeks after the TAC surgical procedure, blood and heart samples were collected. FS in the infected group of WT mice was significantly lower than in mice that received sham operations; however, FS in the uninfected group did not decrease in a similar manner to that in the infected group. Cardiac fibrosis was significantly enhanced in TAC-operated WT mice infected with P. gingivalis (n=14), whereas it was inhibited in TAC-operated TLR-2 KO mice infected with P. gingivalis (n=7). The level of matrix metalloproteinase-2 (MMP-2) mRNA was higher in WT mice infected with P. gingivalis compared with non-infected WT mice. However, the level of MMP-2 mRNA was significantly lower in TLR-2 KO mice compared with that in WT mice. In conclusion, TLR-2 had a critical role in the development of cardiac fibrosis under the conditions of pressure overload and periodontal pathogen infection.
Similar content being viewed by others
Introduction
Myocardial fibrosis is a major compensatory mechanism that occurs in response to pressure overload caused by hypertension and aortic stenosis.1 This remodeling process consists of abnormal changes in the extracellular matrix (ECM) network and hypertrophic changes in cardiac myocytes. Matrix metalloproteinases (MMPs) are a family of extracellular endopeptidases that are designed to maintain the microenvironment of the ECM and have an important role in the cardiac remodeling process.2 It has been reported that MMP-2 mRNA levels significantly increased during pressure-overload cardiac hypertrophy.3, 4 Some experimental trials have been conducted to try to suppress high-pressure-induced myocardial remodeling. An angiotensin receptor blocker, telmisartan, has been shown to alter the balance of MMPs and reduce myocardial fibrosis.5 Another angiotensin receptor blocker, valsartan, when given along with cilnidipine, attenuated myocardial fibrosis via the suppression of oxidative stress and inflammation.6 A mineralocorticoid receptor antagonist, spironolactone, attenuated myocardial fibrosis that was induced by excess aldosterone owing to high salt intake.7 A glucocorticoid receptor blocker also attenuated myocardial fibrosis and reduced cardiac oxidative stress and inflammation.8
Periodontitis is an infectious disease that is induced by several periodontopathic bacteria, including Porphyromonas gingivalis (P. gingivalis) and Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans). Infection with P. gingivalis induces an inflammatory reaction and results in the destruction of gingival connective tissue and in bone resorption.9 MMPs are also involved in the gingival ECM destruction of periodontitis.10, 11 Recently, an association between periodontitis and coronary heart disease has been demonstrated.12 It is also known that periodontal infection may be a contributing risk factor for heart disease.13
Toll-like receptors (TLRs) are pattern-recognition receptors that recognize microbes and lead to inflammation.14 TLRs are selectively upregulated after infection with pathogens occurs.15 P. gingivalis mainly stimulates TLR-2.16 TLR-2 is expressed not only in immune cells but also in cardiovascular cells. TLR-2-mediated inflammation is critically involved in adaptive cardiac hypertrophy during pressure overload. Periodontal pathogens are involved in increasing the plasma levels of inflammatory mediators via TLR-2 during periodontitis, which provides a novel link between periodontitis and an increased risk of cardiovascular disease.17
Recent studies have indicated that periodontopathic bacteria may accelerate the development of myocardial remodeling;18 however, the role of TLRs in the acceleration of pressure-overload-induced myocardial fibrosis during a periodontal bacterial infection has not yet been investigated. Thus the purpose of this study was to investigate the role of TLR-2 in the association between periodontopathic bacterial infection and pressure-overload-induced myocardial fibrosis.
Material and methods
Animals
Eight-to-10-week-old wild-type (WT) C57BL/6 male mice were obtained from CLEA Japan (Tokyo, Japan). The TLR-2 knockout (KO) male mice were obtained from Oriental Yeast (Tokyo, Japan), and the company verified the genetic condition of the mice.19 All experiments conformed to the Guide for the Care and Use of Laboratory Animals of the Tokyo Medical and Dental University (approved numbers 0150166A and 2009-015C2).
Bacterial growth
P. gingivalis strain A7A1-28 was used in this study. The bacteria was grown on blood agar in an anaerobic chamber with 85% N2, 5% H2 and 10% CO2. After incubation at 37 °C for 2–3 days, the bacterial cells were injected into a peptone yeast extract for a 1-week incubation under the same conditions. The purity of the cultures was checked via phase-contrast microscopy. Bacterial cells were in the peptone yeast extract for 1–2 days. Bacterial concentrations were standardized to 108 colony-forming units (CFUs) ml−1.
The subcutaneous chamber model
A chamber (length 10 mm, diameter 5.0 mm) made of stainless wire was implanted subcutaneously into the back of each anesthetized mouse via a dorsal incision. The implanted chambers were used to inject bacteria and induce inflammation. P. gingivalis (0.1 ml of 108 CFUs ml−1) or vehicle (0.1 ml of phosphate-buffered saline) was injected into each chamber to induce inflammation once a week after the transverse aortic constriction (TAC) surgical procedure. Plasma samples were obtained when the mice were killed and the level of anti-P. gingivalis immunoglobulin G (IgG) antibodies in the plasma was determined using an enzyme-linked immunosorbent assay as previously described.20 We used this animal model as a periodontopathic bacteremia model to demonstrate the systemic influence of systemic infections on cardiovascular diseases, such as abdominal aortic aneurysm,21 arteriosclerosis,22 myocardial infarction,23 myocarditis24 and a pressure-overloaded heart.25
Transverse aortic constriction
Two weeks after chamber implantation, TAC surgical procedures were performed in age-matched WT and TLR-2 KO mice, as described previously,18 to induce cardiac pressure overload. The mice were anesthetized with 3.6% chloral hydrate, which was intraperitoneally administered at 0.1 ml per 10 g body weight. A thoracotomy was performed via the second intercostal space at the central upper sternal border to expose the transversal aorta. The ascending aorta was ligated with an overlying blunted 26-gauge needle. Afterward, the needle was immediately removed. Sham-operated mice underwent a similar procedure, but the aortas were not ligated. A tail-cuff system (BP-98A, Softron, Tokyo, Japan) was used to measure blood pressure and heart rate immediately before the TAC operation and also 1 week later.
Echocardiography
An echocardiography exam was performed using ultrasound equipment (Nemio, Toshiba, Tokyo, Japan) 1 and 4 weeks after the TAC procedure while mice were anesthetized with 3.6% chloral hydrate, which was administered intraperitoneally at 0.1 ml per 10 g body weight. The M-mode view was used to measure the left ventricular (LV) dimensions. The LV end-diastolic dimension (LVEDD) and the LV end-systolic dimension (LVESD) were obtained using M-mode tracings of the LV at the papillary muscle level. Fractional shortening (FS) was calculated as (LVEDD−LVESD)/LVEDD × 100.
Organ weight and heart dissection
Four weeks after the TAC procedure, the whole body and heart were weighed. Hearts were then cut at the level of the atrial appendage to divide them into their atrial and ventricular parts. Ventricular parts were then cut into three sections across the septum.
Histopathology
Formalin-fixed (10%), paraffin-embedded hearts were sectioned at 5-μm thickness and stained according to the Mallory method. To quantify the fibrosis area, blue-stained collagen fibers in the Mallory-stained samples were traced using the Image-Pro Express software (Media Cybernetics, Silver Spring, MD, USA). Then the ratio of fibrosis per whole myocardium area was calculated.
RNA isolation and reverse transcriptase-PCR
Total RNA was extracted from the heart tissue using a TRIzol reagent (Invitrogen/Life Technologies, CA, USA) according to the manufacturer’s instructions. Reverse transcriptase-PCR was performed to determine the mRNA levels of MMP-2 (Assay ID: Mm00439498 m1; Applied Biosystems, Tokyo, Japan), MMP-9 (Assay ID: Mm00600163 m1; Applied Biosystems), tumor necrosis factor (Assay ID: Mm00443260 g1; Applied Biosystems), transforming growth factor-beta1 (Assay ID: Mm01178820 m1; Applied Biosystems) and atrial natriuretic peptide (Assay ID: Mm01255747; Applied Biosystems). The mRNA expression levels of the targeted genes were normalized to 18s rRNA. Quantitative data were calculated using the comparative CT (ΔΔCT) method and the mRNA expression was normalized to native samples.
Statistical analysis
All data were displayed as the mean±s.e.m. Differences between multiple groups were determined using analyses of variance, followed by Tukey–Kramer tests (Stat View, SAS Institute Japan, Tokyo, Japan). P<0.05 was considered to be statistically significant.
Results
Antibacterial antibodies
The levels of anti-P. gingivalis IgG antibody were significantly increased in the P. gingivalis-infected groups. The injection of vehicle (phosphate-buffered saline) had no significant effect on the levels of anti-P. gingivalis IgG antibody (Table 1).
Physiological measurements
The echocardiograms indicated that FS of P. gingivalis-infected TAC mice was lower than that of sham mice in the WT groups. In WT or TLR-2 KO mice that underwent TAC operations, FS was comparable between the non-infected and P. gingivalis-infected mice (Figure 1 and Table 2). Blood pressure and heart rate were comparable among the groups (data not shown).
Organ weight
The heart-to-body weight ratio tended to be higher in TAC-operated WT mice compared with sham-operated mice. However, there were no statistically significant differences among the groups (Table 3).
Histopathology
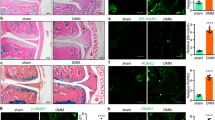
A transverse cross-section from each of the hearts that underwent Mallory staining was evaluated (Figures 2a and b). In WT mice, a higher amount of expanded myocardial fibrosis was observed in TAC-operated mice compared with sham-operated mice. P. gingivalis-infected TAC mice had more fibrosis than non-infected TAC mice in the WT groups. In sham-operated mice, P. gingivalis injection had no pathological effects on fibrosis (Figure 2c). It is important to note that the area of fibrosis was significantly less in TLR-2 KO mice compared with WT mice that had undergone both a TAC procedure and P. gingivalis injections. However, when mice had only undergone a TAC procedure, the area of fibrosis in WT and TLR-2 KO mice was comparable (Figures 2d and e).
Histopathology. Representative histopathological findings using Mallory staining (a), low-power microscopic field, scale bars of 500 μm; (b), high-power microscopic field, scale bars of 100 μm) and quantitative data are presented (c), comparisons in the WT groups; (d), comparisons in the LR2-KO groups; (e), comparisons between the WT and KO groups, WT mice: n=14, TLR-2 KO mice: n=7). PBS, phosphate-buffered saline; P.g., Porphyromonas gingivalis; TAC, transverse aortic constriction; TLR-2 KO, Toll-like receptor-2 knockout; WT, wild type. *P<0.05. A full color version of this figure is available at the Hypertension Research journal online.
Reverse transcriptase-PCR
The MMP-2 mRNA levels in the TLR-2 KO mice that received P. gingivalis injections was significantly lower than that in WT mice but was comparable between WT and TLR-2 KO mice that did not receive P. gingivalis injections (Figure 3a). A similar tendency was observed in the MMP-9 mRNA levels, but there was no statistically significant difference (Figure 3b).
The levels of tumor necrosis factor mRNA in the TLR-2 KO mice that received P. gingivalis injections were significantly lower than in WT mice, while levels were comparable between WT and TLR-2 KO mice that were not administered P. gingivalis (Figure 4a). The levels of transforming growth factor-beta1 mRNA in the TLR-2 KO mice were significantly lower than in WT mice (Figure 4b). The atrial natriuretic peptide mRNA levels in the TLR-2 KO mice that did not receive P. gingivalis were significantly lower than in WT mice but were comparable between WT and TLR-2 KO mice that did receive P. gingivalis injections (Figure 4c).
Discussion
It has been reported that periodontitis is associated with many systemic diseases, such as diabetes mellitus, cardiovascular disease and brain infarction. Many studies have demonstrated the relationship between periodontal disease and heart disease.12, 13, 25 The risk of atherosclerotic cardiovascular disease have been shown to be higher in patients with periodontitis compared with patients without it.26 It has been reported that infection with A. actinomycetemcomitans, a periodontopathic pathogen, worsened pressure-overload-induced cardiac hypertrophy and fibrosis in mice.18 However, the precise mechanisms of how periodontitis and cardiac fibrosis are connected has not yet been clarified. The present investigation revealed that injections of P. gingivalis increased pressure-overload-induced myocardial fibrosis. It has been demonstrated that infection with periodontal pathogens causes systemic inflammation27 and that inflammation is positively correlated with left ventricular hypertrophy.28 In the P. gingivalis-infected groups in our study, the levels of anti-P. gingivalis IgG antibodies were elevated. Therefore, we considered these mice to be infected with P. gingivalis. Although the subcutaneous chamber model may be different from an actual periodontal infection, we considered it to be similar in terms of systemic inflammation. In this study, we hypothesized that systemic inflammation induced by P. gingivalis infection would worsen cardiac fibrosis during pressure overload.
In this report, pressure-overload-induced cardiac fibrosis was attenuated in P. gingivalis-infected TLR-2 KO mice compared with P. gingivalis-infected WT mice. TLRs are critical pattern-recognition receptors that recognize pathogen-associated molecular patterns.29 Many periodontal bacteria stimulate TLR-2 and periodontitis increases TLR-2 gene expression in gingival tissues. TLR recognition triggers the release of inflammatory cytokines and other downstream factors for the purpose of host defense. Higashikuni et al.30 revealed that TLR-2 KO mice showed reduced cardiac hypertrophy and fibrosis compared with WT mice 2 weeks after TAC. In vitro experiments demonstrated that TLR2 signaling induced cardiomyocyte hypertrophy and fibroblast proliferation via nuclear factor-κB (NF-kappaB) activation and interleukin-1beta upregulation.30 Because NF-kappaB has a central role in inflammation, we previously showed that inhibition of IκB phosphorylation prevents load-induced cardiac fibrosis in mice via MMP suppression.31 Wei et al.32 also showed that inhibition of NF-kappaB in cardiac fibroblasts restored miR-26a expression, which attenuated collagen I, and also restored connective tissue growth factor gene expression in the presence of Ang II, thereby providing a regulatory feedback mechanism in cardiac fibrosis. Thus the TLR-2 to NF-κB stream is critical for the development of myocardial fibrosis during pressure overload. Monaco et al.33 reported that blocking TLR-2 significantly reduced MMP production in cultured human atheroma cells.
MMP-2 activity has been shown to be higher in the human left ventricular myocardium during heart failure.34 An imbalance in the function of MMPs and the myocardial tissue inhibitor of MMPs occurs during heart disease and leads to adverse ECM remodeling.35 The serum levels of MMP-2 have been shown to be higher in patients with hypertensive heart disease and myocardial infarction.36 This indicates that, under these conditions, MMP activation is related to the cardiac remodeling process. Matsusaka et al.4 reported that MMP-2 KO TAC mice had less myocyte hypertrophy and interstitial fibrosis than WT TAC mice. They concluded that MMP-2 had an important role in pressure-overload-induced LV hypertrophy and dysfunction. Furthermore, the expression of MMP-2 was not enhanced in the myocardium of TLR-2 KO mice in our study.
Periodontal pathogens increase MMPs activity. Andrian et al.37 reported on the ability of P. gingivalis to regulate MMP and tissue inhibitor of MMP production by oral cells, a phenomenon that might contribute to tissue damage. MMP-2 is involved in physiological tissue remodeling and the pathological ECM degradation that lead to the pathogenesis of periodontal disease.38 The levels of MMPs have been to be significantly decreased after periodontal therapies.39 These results may provide support for our findings that periodontal pathogen-induced MMP-2 worsened myocardial fibrosis in TAC hearts. Based on the results from the current study and from previous reports, we speculate that P. gingivalis is recognized by TLR-2-induced systemic inflammatory reactions that result from cardiac fibrosis. As we showed, some inflammatory factors were suppressed in TLR-2 KO mice. Further research is needed to clarify this mechanism.
In conclusion, TLR-2 is involved in the link between periodontitis and cardiac fibrosis. Ours is the first report that shows that P. gingivalis promotes cardiac fibrosis via TLR-2. TLR-2 is important for recognizing P. gingivalis and for the subsequent promotion of systemic inflammation and enhancement of cardiac fibrosis induced by pressure overload.
References
Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K . Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension 2006; 47: 887–893.
Lee SD, Wu CC, Chang YC, Chang SH, Wu CH, Wu JP, Hwang JM, Kuo WW, Liu JY, Huang CY . Porphyromonas gingivalis-induced cellular hypertrophy and MMP-9 activity via different signaling pathways in H9c2 cardiomyoblast cells. J Periodontol 2006; 77: 684–691.
Peterson JT, Hallak H, Johnson L, Li H, O'Brien PM, Sliskovic DR, Bocan TM, Coker ML, Etoh T, Spinale FG . Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation 2001; 103: 2303–2309.
Matsusaka H, Ide T, Matsushima S, Matsushima S, Ikeuchi M, Kubota T, Sunagawa K, Kinugawa S, Tsutsui H . Targeted deletion of matrix metalloproteinase 2 ameliorates myocardial remodeling in mice with chronic pressure overload. Hypertension 2006; 47: 711–717.
Zhang Y, Shao L, Ma A, Guan G, Wang J, Wang Y, Tian G . Telmisartan delays myocardial fibrosis in rats with hypertensive left ventricular hypertrophy by TGF-β1/Smad signal pathway. Hypertens Res 2014; 37: 43–49.
Nagasawa K, Takahashi K, Matsuura N, Takatsu M, Hattori T, Watanabe S, Harada E, Niinuma K, Murohara T, Nagata K . Comparative effects of valsartan in combination with cilnidipine or amlodipine on cardiac remodeling and diastolic dysfunction in Dahl salt-sensitive rats. Hypertens Res 2015; 38: 39–47.
Hattori T, Murase T, Sugiura Y, Nagasawa K, Takahashi K, Ohtake M, Ohtake M, Miyachi M, Murohara T, Nagata K . Effects of salt status and blockade of mineralocorticoid receptors on aldosterone-induced cardiac injury. Hypertens Res 2014; 37: 125–133.
Takeshita Y, Watanabe S, Hattori T, Nagasawa K, Matsuura N, Takahashi K, Murohara T, Nagata K . Blockade of glucocorticoid receptors with RU486 attenuates cardiac damage and adipose tissue inflammation in a rat model of metabolic syndrome. Hypertens Res 2015; 38: 741–750.
Buchwald S, Kocher T, Biffar R, Harb A, Holtfreter B, Meisel P . Tooth loss and periodontitis by socio-economic status and inflammation in a longitudinal population-based study. J Clin Periodontol 2013; 40: 203–211.
Bildt MM, Bloemen M, Kuijpers-Jagtman AM, Von den Hoff JW . Collagenolytic fragments and active gelatinase complexes in periodontitis. J Periodontol 2008; 79: 1704–1711.
Dong W, Xiang J, Li C, Cao Z, Huang Z . Increased expression of extracellular matrix metalloproteinase inducer is associated with matrix metalloproteinase-1 and -2 in gingival tissues from patients with periodontitis. J Periodontal Res 2009; 44: 125–132.
Dietrich T, Jimenez M, Krall Kaye EA, Vokonas PS, Garcia RI . Age-dependent associations between chronic periodontitis/edentulism and risk of coronary heart disease. Circulation 2008; 117: 1668–1674.
Tonetti MS, Van Dyke TE, working group 1 of the joint EFP/AAP workshop. Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol 2013; 84: S24–S29.
Lien E, Ingalls RR . Toll-like receptors. Crit Care Med 2002; 30: S1–S11.
Wang T, Lafuse WP, Zwilling BS . Regulation of toll-like receptor 2 expression by macrophages following mycobacterium avium infection. J Immunol 2000; 165: 6308–6313.
Kikkert R, Laine ML, Aarden LA, van Winkelhoff AJ . Activation of toll-like receptors 2 and 4 by gram-negative periodontal bacteria. Oral Microbiol Immunol 2007; 22: 145–151.
Assinger A, Laky M, Badrnya S, Esfandeyari A, Volf I . Periodontopathogens induce expression of CD40l on human platelets via TLR2 and TLR4. Thromb Res 2012; 130: e73–e78.
Sekinishi A, Suzuki J, Aoyama N, Ogawa M, Watanabe R, Kobayashi N, Hanatani T, Ashigaki N, Hirata Y, Nagai R, Izumi Y, Isobe M . Periodontal pathogen Aggregatibacter actinomycetemcomitans deteriorates pressure overload-induced myocardial hypertrophy in mice. Int Heart J 2012; 53: 324–330.
Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S . Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity 1999; 11: 443–451.
Kojima T, Yano K, Ishikawa I . Relationship between serum antibody levels and subgingival colonization of Porphyromonas gingivalis in patients with various types of periodontitis. J Periodontol 1997; 68: 618–625.
Aoyama N, Suzuki J, Ogawa M, Watanabe R, Kobayashi N, Hanatani T, Ashigaki N, Sekinishi A, Izumi Y, Isobe M . Toll-like receptor-2 plays a fundamental role in the periodontal bacteria-accelerated abdominal aortic aneurysms. Circ J 2013; 77: 1565–1573.
Kobayashi N, Suzuki J, Ogawa M, Aoyama N, Komuro I, Izumi Y, Isobe M . Porphyromonas gingivalis promotes neointimal formation after arterial injury through toll-like receptor 2 signaling. Heart Vessels 2014; 29: 542–549.
Hanatani T, Suzuki J, Ogawa M, Aoyama N, Kobayashi N, Hirata Y, Nagai R, Izumi Y, Isobe M . A periodontal pathogen Aggregatibacter actinomycetemcomitans deteriorates ventricular remodeling after myocardial infarction in mice. Int Heart J 2012; 53: 253–256.
Ashigaki N, Suzuki J, Ogawa M, Watanabe R, Aoyama N, Kobayashi N, Hanatani T, Sekinishi A, Zempo H, Tada Y, Takamura C, Wakayama K, Hirata Y, Nagai R, Izumi Y, Isobe M . Periodontal bacteria aggravate experimental autoimmune myocarditis in mice. Am J Physiol Heart Circ Physiol 2013; 304: H740–H748.
Holmlund A, Hedin M, Pussinen PJ, Lerner UH, Lind L . Porphyromonas gingivalis Pg a possible link between impaired oral health and acute myocardial infarction. Int J Cardiol 2011; 148: 148–153.
Dietrich T, Sharma P, Walter C, Weston P, Beck J . The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J Clin Periodontol 2013; 40 (Suppl 14): S70–S84.
Hajishengallis G . Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol 2015; 15: 30–44.
Smeets PJ, Teunissen BE, Willemsen PH, van Nieuwenhoven FA, Brouns AE, Janssen BJ, Cleutjens JP, Staels B, van der Vusse GJ, van Bilsen M . Cardiac hypertrophy is enhanced in PPAR alpha-/- mice in response to chronic pressure overload. Cardiovasc Res 2008; 78: 79–89.
Qian C, Cao X . Regulation of toll-like receptor signaling pathways in innate immune responses. Ann NY Acad Sci 2013; 1283: 67–74.
Higashikuni Y, Tanaka K, Kato M, Nureki O, Hirata Y, Nagai R, Komuro I, Sata M . Toll-like receptor-2 mediates adaptive cardiac hypertrophy in response to pressure overload through interleukin-1beta upregulation via nuclear factor κB activation. J Am Heart Assoc 2013; 2: e000267.
Tanaka T, Suzuki J, Ogawa M, Itai A, Hirata Y, Nagai R, Isobe M . Inhibition of I kappaB phosphorylation prevents load-induced cardiac dysfunction in mice. Am J Physiol Heart Circ Physiol 2012; 303: H1435–H1445.
Wei C, Kim IK, Kumar S, Jayasinghe S, Hong N, Castoldi G, Catalucci D, Jones WK, Gupta S . NF-kappaB mediated miR-26a regulation in cardiac fibrosis. J Cell Physiol 2013; 228: 1433–1442.
Monaco C, Gregan SM, Navin TJ, Foxwell BM, Davies AH, Feldmann M . Toll-like receptor-2 mediates inflammation and matrix degradation in human atherosclerosis. Circulation 2009; 120: 2462–2469.
Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ . A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation 2000; 102: 1944–1949.
Kandalam V, Basu R, Moore L, Fan D, Wang X, Jaworski DM, Oudit GY, Kassiri Z . Lack of tissue inhibitor of metalloproteinases 2 leads to exacerbated left ventricular dysfunction and adverse extracellular matrix remodeling in response to biomechanical stress. Circulation 2011; 124: 2094–2105.
Squire IB, Evans J, Ng LL, Loftus IM, Thompson MM . Plasma MMP-9 and MMP-2 following acute myocardial infarction in man: correlation with echocardiographic and neurohumoral parameters of left ventricular dysfunction. J Card Fail 2004; 10: 328–333.
Andrian E, Mostefaoui Y, Rouabhia M, Grenier D . Regulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases by Porphyromonas gingivalis in an engineered human oral mucosa model. J Cell Physiol 2007; 211: 56–62.
Sorsa T, Tjäderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, Golub LM, Brown DL, Mäntylä P . Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med 2006; 38: 306–321.
Tüter G, Kurtiş B, Serdar M, Aykan T, Okyay K, Yücel A, Toyman U, Pinar S, Cemri M, Cengel A, Walker SG, Golub LM . Effects of scaling and root planing and sub-antimicrobial dose doxycycline on oral and systemic biomarkers of disease in patients with both chronic periodontitis and coronary artery disease. J Clin Periodontol 2007; 34: 673–681.
Acknowledgements
We thank Ms Noriko Tamura and Ms Yasuko Matsuda for their excellent technical assistance. This work was supported by the Japan Society for the Promotion of Science (ID: 15K20616), the Mitsui Life Social Welfare Foundation, the Daiwa Security Health Foundation, the Mitsui Sumitomo Marine Welfare Foundation, the Institute of Geriatric Dentistry Foundation, the Institute of St Luka Life Science Foundation, the Health Welfare Foundation, the Taiyo Life Welfare Foundation, the 8020 Promotion Foundation, the Terumo Science Foundation, the Pfizer Health Research Foundation, the General Health Promotion Foundation, the Health Science Center Foundation, the Kobayashi International Scholarship Foundation and the Suzuken Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kaneko, M., Suzuki, Ji., Aoyama, N. et al. Toll-like receptor-2 has a critical role in periodontal pathogen-induced myocardial fibrosis in the pressure-overloaded murine hearts. Hypertens Res 40, 110–116 (2017). https://doi.org/10.1038/hr.2016.117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2016.117
Keywords
This article is cited by
-
Short-term effect of ligature-induced periodontitis on cardiovascular variability and inflammatory response in spontaneously hypertensive rats
BMC Oral Health (2021)
-
Cardiovascular and Autonomic Dysfunction in Murine Ligature-Induced Periodontitis
Scientific Reports (2020)
-
Periodontitis and myocardial hypertrophy
Hypertension Research (2017)