Abstract
Megalencephalic leukoencephalopathy with subcortical cysts (MLC) is an autosomal recessive neurological disorder manifesting early onset macrocephaly and delayed-onset neurological deterioration. Characteristic radiological findings revealed by brain magnetic resonance imaging are the most important factors for obtaining a clinical diagnosis. In this study, we analyzed the causative gene, MLC1, in seven unrelated Japanese patients. The most common mutation in our study was p.S93L; this mutation was observed in 11 alleles (78.6%). The second most common mutation, p.A275D, was observed in two alleles (14.3%). A novel single-nucleotide deletion, c.578delG (p.V194Sfs*2), was identified in one allele. As the clinical severities of patients with MLC were variable even among those sharing identical genotypes, this condition may be modified by environmental factors, modifier genes or epigenetic factors.
Similar content being viewed by others
Megalencephalic leukoencephalopathy with subcortical cysts (MLC, MIM #604004) is an autosomal recessive neurological disorder first described by van der Knaap et al.1 that is characterized by early onset macrocephaly and delayed-onset neurological deterioration.1 Patients with MLC show macrocephaly during the first year of life, followed by slowly progressive deterioration of motor functions with ataxia and spasticity. In such cases, brain magnetic resonance imaging (MRI) shows diffuse signal abnormality in the white matter of the cerebral hemisphere, and subcortical cysts are often observed in the anterior-temporal region and/or in the front-parietal area. The causative gene, MLC1 (MIM #605908), was first identified in 2001 and maps to chromosome band 22q13.33; this gene contains 12 exons.2 MLC1 mutations have been observed in 75% of patients with MLC.3 Herein, we report the result of an ongoing study to obtain a genetic diagnosis for MLC1.
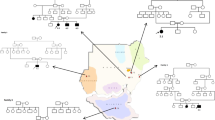
This study was approved by the Ethics Committee of Tokyo Women’s Medical University. After obtaining written informed consent from patients or their families, blood samples were obtained from patients with a clinical diagnosis of MLC. The diagnosis was made based on previously defined criteria.1,4 Genomic DNA was extracted from blood samples using the QIAamp DNA Extraction Kit (QIAGEN, Hamburg, Germany). Parental samples were also obtained to determine inheritance. All exons of MLC1 were genotyped using standard Sanger sequencing.
We identified three types of MLC1 mutations in seven unrelated Japanese patients with MLC (Table 1). Eleven alleles contained the c.278C>T (p.S93L) missense mutation (Supplementary Figure 1) in exon 4 (78.6%), and all patients had p.S93L in either of the alleles (Table 1). Patients 1–4 showed homozygous patterns of p.S93L, and the remaining three patients were compound heterozygous for the mutation and one of other mutations. This mutation has been previously reported in individuals from Japan, Turkey and Finland.2,5,6 Tsujino et al.6 reported that p.S93L is a common mutation in Japanese patients with MLC, with 85.7% of patients and 71.4% alleles showing this mutation.6 This finding was also confirmed in the present study. Although we did not analyze parental origins in patients 1–4 due to lack of parental samples, parental consanguinities in patients 1–3 and the high frequency of p.S93L suggest that these patients would be homozygous for p.S93L.
The second most common mutation, c.824C>A (p.A275D) in exon 10 (Supplementary Figure 1), was observed in two alleles (14.3%) from patients 5 and 7 (Table 1). Compound heterozygosity was confirmed in these two patients because p.S93L and p.A275D were identified in either of the parents independently. Although this mutation was reported previously by Montagna et al.,7 this is the first report in Japanese patients.
A single-nucleotide deletion, c.578delG (p.V194Sfs*2) (Supplementary Figure 1), was identified in exon 7 in patient 6. To the best of our knowledge, this is a novel mutation. The mother of patient 6 was a carrier of p.S93L (heterozygous of p.S93L); however, the origin of c.578delG was unknown due to lack of a sample from his father. This deletion causes a frameshift mutation and creates a premature termination codon, leading to nonsense-mediated decay. As a consequence, this likely leads to a loss of function of MLC1. Such single-nucleotide deletions are rare in MLC1.
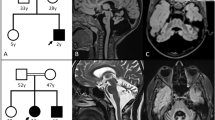
In the present study, all patients showed brain MRI abnormalities consistent with diffuse white matter abnormalities (Figure 1). Although most of the patients showed detectable subcortical cysts, particularly in anterior-temporal regions (Figure 1), some were too small to be detected in the regular images. The MLC patients could be classified into two groups according to age, that is, infant patients and patients older than the teenage years. Two infant patients were diagnosed with MLC as a result of examination for macrocephaly; no neurological findings were observed in either of these two patients at the time of genetic diagnosis.
Brain magnetic resonance imaging findings. (a and b) Patient 1 examined at 55 years of age. The T1-weighted sagittal image (a) shows a subcortical cyst in the anterior-temporal region, and diffuse volume loss of the cerebrum is noted (b). (c and d) Patient 2 at 51 years of age. Although subcortical cysts are too small to be detected in the anterior-temporal regions (c), mild volume loss of the cerebrum is detectable (d). (e and f) Patient 3 at 16 years of age. (g and h) and patient 4 at 30 years of age. The T1-weighted axial image (g) shows subcortical cysts in the anterior-temporal regions. (i and j) Patient 5 at 18 years of age. An asymmetric subcortical cyst in the right anterior-temporal regions is noted in the T1-weighted axial image (i). (k and l) Patient 6 at 11 months of age. (m and n) patient 7 at 9 months of age. The T2-weighted axial images indicate high intensity in the white matter in all patients (b–f, h and j–n). Subcortical cysts in the anterior-temporal regions are detectable in some of the T2-weighted axial images (e and m).
In comparison, the older patients showed impaired motor and/or intellectual disability and required supports for daily life. Macrocephaly was observed in only two of the five older patients (2/5). The clinical courses and prognoses of these patients were variable. The onset of MLC in the four patients with the p.S93L homozygous mutation was variable, ranging from 15 months to 41 years. Patient 1, who was homozygous for p.S93L, was severely impaired and bedridden from the age of 4. On the other hand, patient 2 showed only mild cognitive and memory deficit and suffered seizures followed by weakness of the extremities at 47 years of age. This type of patient with a good long-term prognosis was also reported by Koyama et al.8 Therefore, this may not necessarily indicate that p.S93L is the mutation causing severe neurological prognosis.
In MLC patients, provoked events are often observed after high fever and head trauma. We observed such provoked events in three patients (Table 1). Because the clinical severities of patients with MLC varied even among patients sharing identical genotypes, disease prognosis may be modified by environmental factors including fever, head trauma, unknown modifier genes, and epigenetic factors.
References
References
van der Knaap MS, Barth PG, Stroink H, van Nieuwenhuizen O, Arts WF, Hoogenraad F et al. Leukoencephalopathy with swelling and a discrepantly mild clinical course in eight children. Ann Neurol 1995; 37: 324–334.
Leegwater PA, Yuan BQ, van der Steen J, Mulders J, Konst AA, Boor PK et al. Mutations of MLC1 (KIAA0027), encoding a putative membrane protein, cause megalencephalic leukoencephalopathy with subcortical cysts. Am J Hum Genet 2001; 68: 831–838.
Ilja Boor PK, de Groot K, Mejaski-Bosnjak V, Brenner C, van der Knaap MS, Scheper GC et al. Megalencephalic leukoencephalopathy with subcortical cysts: an update and extended mutation analysis of MLC1. Hum Mutat 2006; 27: 505–512.
van der Knaap MS, Boor I, Estevez R . Megalencephalic leukoencephalopathy with subcortical cysts: chronic white matter oedema due to a defect in brain ion and water homoeostasis. Lancet Neurol 2012; 11: 973–985.
Saijo H, Nakayama H, Ezoe T, Araki K, Sone S, Hamaguchi H et al. A case of megalencephalic leukoencephalopathy with subcortical cysts (van der Knaap disease): molecular genetic study. Brain Dev 2003; 25: 362–366.
Tsujino S, Kanazawa N, Yoneyama H, Shimono M, Kawakami A, Hatanaka Y et al. A common mutation and a novel mutation in Japanese patients with van der Knaap disease. J Hum Genet. 2003; 48: 605–608.
Montagna G, Teijido O, Eymard-Pierre E, Muraki K, Cohen B, Loizzo A et al. Vacuolating megalencephalic leukoencephalopathy with subcortical cysts: functional studies of novel variants in MLC1. Hum Mutat 2006; 27: 292.
Koyama S, Kawanami T, Arawaka S, Wada M, Kato T . A Japanese adult case of megalencephalic leukoencephalopathy with subcortical cysts with a good long-term prognosis. Intern Med 2012; 51: 503–506.
Data Citations
Yamamoto, Toshiyuki HGV Database (2014) http://dx.doi.org/10.6084/m9.figshare.hgv.515
Yamamoto, Toshiyuki HGV Database (2014) http://dx.doi.org/10.6084/m9.figshare.hgv.517
Yamamoto, Toshiyuki HGV Database (2014) http://dx.doi.org/10.6084/m9.figshare.hgv.519
Yamamoto, Toshiyuki HGV Database (2014) http://dx.doi.org/10.6084/m9.figshare.hgv.521
Acknowledgements
We acknowledge the Collaborative Research Supporting Committee of the Japanese Society of Child Neurology (14-3) for promoting this study. This work was supported by a Grant-in-Aid for Scientific Research from Health Labor Sciences Research Grants from the Ministry of Health, Labor and Welfare, Japan (TY).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information for this article can be found on the Human Genome Variation website (http://www.nature.com/hgv)
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Shimada, S., Shimojima, K., Masuda, T. et al. MLC1 mutations in Japanese patients with megalencephalic leukoencephalopathy with subcortical cysts. Hum Genome Var 1, 14019 (2014). https://doi.org/10.1038/hgv.2014.19
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/hgv.2014.19
This article is cited by
-
Novel variants causing megalencephalic leukodystrophy in Sudanese families
Journal of Human Genetics (2022)