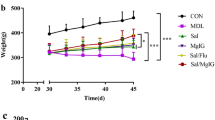
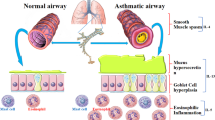
Abstract
Asthma is a multifactorial disease that is influenced by the interaction of genetic and environmental factors. Because of its complex nature, there is no cure for asthma currently. Instead, reliever and controller medications are used to treat asthma. Unfortunately, conventional treatments do not work in some severe cases of asthma. In addition, there may be adverse, systemic effects of long-term treatment with high-dose inhaled corticosteroids (ICSs) as a controller medication. Therefore, we attempted to develop a novel combination therapy for asthma. Our regimen included dexamethasone as a controller medication and vitamin D binding protein (VDBP) small interfering RNA (siRNA) as a novel target therapeutic. The dexamethasone moiety of DEXA-PEI (dexamethasone-conjugated polyethylenimine) was used as an ICS, combined with anti-VDBP treatment via delivery of VDBP siRNA, using DEXA-PEI as a siRNA carrier molecule. Treatment with DEXA-PEI/VDBP siRNA effectively reduced the ovalbumin sensitization/challenge-induced enhancement of airway inflammation, goblet cell hyperplasia and expression of interleukin (IL)-4, IL-13 and CCL11. These findings suggest that the DEXA-PEI/VDBP siRNA can be developed as a potent asthma therapeutic by dose-reducing ICSs and using a multitarget therapeutic method.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hamid Q, Tulic M . Immunobiology of asthma. Annu Rev Physiol 2009; 71: 489–507.
Reddel HK, Hurd SS, Fitzgerald JM . World Asthma Day. GINA 2014: a global asthma strategy for a global problem. Int J Tuberc Lung Dis 2014; 18: 505–506.
Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J 2008; 31: 143–178.
Patel M, Pilcher J, Pritchard A, Perrin K, Travers J, Shaw D et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerbations: a randomised controlled trial. Lancet Resp Med 2013; 1: 32–42.
Morrison D, Capewell S, Reynolds SP, Thomas J, Ali NJ, Read GF et al. Testosterone levels during systemic and inhaled corticosteroid-therapy. Resp Med 1994; 88: 659–663.
Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy 2004; 59: 469–478.
Mealey FH, Kenyon NJ, Avdalovic MV, Louie S . Difficult-to-control asthma in adults. Am J Med 2007; 120: 760–763.
Hodgson D, Anderson J, Reynolds C, Meakin G, Bailey H, Pavord I et al. A randomised controlled trial of small particle inhaled steroids in refractory eosinophilic asthma (SPIRA). Thorax 2015; 70: 559–565.
ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH . "Refractory" eosinophilic airway inflammation in severe asthma - effect of parenteral corticosteroids. Am J Resp Crit Care 2004; 170: 601–605.
Zimmermann N, Hershey GK, Foster PS, Rothenberg ME . Chemokines in asthma: cooperative interaction between chemokines and IL-13. J Allergy Clin Immun 2003; 111: 227–242.
Barnes PJ . The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest 2008; 118: 3546–3556.
Ogawa Y, Calhoun WJ . The role of leukotrienes in airway inflammation. J Allergy Clin Immun 2006; 118: 789–798.
Catley MC, Coote J, Bari M, Tomlinson KL . Monoclonal antibodies for the treatment of asthma. Pharmacol Therapeut 2011; 132: 333–351.
Corren J . Inhibition of interleukin-5 for the treatment of eosinophilic diseases. Discov Med 2012; 71: 305–312.
Cook ML, Bochner BS . Update on biological therapeutics for asthma. World Allergy Organ J 2010; 3: 188–194.
Lee SH, Kim KH, Kim JM, Yoon SH, Kim TH, Park SW et al. Relationship between group-specific component protein and the development of asthma. Am J Resp Crit Care 2011; 184: 528–536.
Kim HA, Park JH, Lee S, Choi JS, Rhim T, Lee M . Combined delivery of dexamethasone and plasmid DNA in an animal model of LPS-induced acute lung injury. J Control Release 2011; 156: 60–69.
Yoder MC, Chua R, Tepper R . Effect of dexamethasone on pulmonary inflammation and pulmonary-function of ventilator-dependent infants with bronchopulmonary dysplasia. Am Rev Respir Dis 1991; 143: 1044–1048.
Kumar RK, Herbert C, Thomas PS, Wollin L, Beume R, Yang M et al. Inhibition of inflammation and remodeling by roflumilast and dexamethasone in murine chronic asthma. J Pharmacol Exp Ther 2003; 307: 349–355.
Elwood W, Lotvall JO, Barnes PJ, Chung KF . Effect of dexamethasone and cyclosporine-A on allergen-induced airway hyperresponsiveness and inflammatory cell responses in sensitized Brown-Norway rats. Am Rev Respir Dis 1992; 145: 1289–1294.
Bae YM, Choi H, Lee S, Kang SH, Kim YT, Nam K et al. Dexamethasone-conjugated low molecular weight polyethylenimine as a nucleus-targeting lipopolymer gene carrier. Bioconjugate Chem 2007; 18: 2029–2036.
Torres R, Picado C, de Mora E . Use of the mouse to unravel allergic asthma: a review of the pathogenesis of allergic asthma in mouse models and its similarity to the condition in humans. Arch Bronconeumol 2005; 41: 141–152.
Tran TH, Rastogi R, Shelke J, Amiji MM . Modulation of macrophage functional polarity towards anti-inflammatory phenotype with plasmid dna delivery in CD44 targeting hyaluronic acid nanoparticles. Sci Rep 2015; 5: 16632.
Kim H, Kim HA, Bae YM, Choi JS, Min M . Dexamethasone-conjugated polyethylenimine as an efficient gene carrier with an anti-apoptotic effect to cardiomyocytes. J Gene Med 2009; 11: 515–522.
Choi M, Lee M, Rhim T . Dexamethasone-conjugated polyethylenimine/MIF siRNA complex regulation of particulate matter-induced airway inflammation. Biomaterials 2013; 34: 7453–7461.
Ahn MH, Kang CM, Park CS, Park SJ, Rhim T, Yoon PO et al. Titanium dioxide particle-induced goblet cell hyperplasia: association with mast cells and IL-13. Respir Res 2005; 6: 34.
Choi JS, Ko KS, Park JS, Kim YH, Kim SW, Lee M . Dexamethasone conjugated poly(amidoamine) dendrimer as a gene carrier for efficient nuclear translocation. Int J Pharm 2006; 320: 171–178.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. NRF-2014R1A2A2A01006740). MC was partly supported by NRF-2012M3A9D1054451.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Choi, M., Gu, J., Lee, M. et al. A new combination therapy for asthma using dual-function dexamethasone-conjugated polyethylenimine and vitamin D binding protein siRNA. Gene Ther 24, 727–734 (2017). https://doi.org/10.1038/gt.2017.83
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2017.83
This article is cited by
-
Recent advances in vitamin D implications in chronic respiratory diseases
Respiratory Research (2022)