Abstract
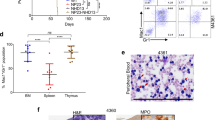
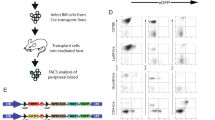
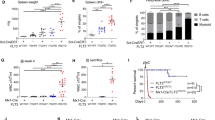
Artemis is a factor of the non-homologous end joining pathway involved in DNA double-strand break repair that has a critical role in V(D)J recombination. Mutations in DCLRE1C/ARTEMIS gene result in radiosensitive severe combined immunodeficiency in humans owing to a lack of mature T and B cells. Given the known drawbacks of allogeneic hematopoietic stem cell transplantation (HSCT), gene therapy appears as a promising alternative for these patients. However, the safety of an unregulated expression of Artemis has to be established. We developed a transgenic mouse model expressing human Artemis under the control of the strong CMV early enhancer/chicken beta actin promoter through knock-in at the ROSA26 locus to analyze this issue. Transgenic mice present a normal development, maturation and function of T and B cells with no signs of lymphopoietic malignancies for up to 15 months. These results suggest that the over-expression of Artemis in mice (up to 40 times) has no deleterious effects in early and mature lymphoid cells and support the safety of gene therapy as a possible curative treatment for Artemis-deficient patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Lieber MR . The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem 2010; 79: 181–211.
Ochi T, Blackford AN, Coates J, Jhujh S, Mehmood S, Tamura N et al. DNA repair. PAXX, a paralog of XRCC4 and XLF, interacts with Ku to promote DNA double-strand break repair. Science 2015; 347: 185–188.
Xing M, Yang M, Huo W, Feng F, Wei L, Jiang W et al. Interactome analysis identifies a new paralogue of XRCC4 in non-homologous end joining DNA repair pathway. Nat Commun 2015; 6: 6233.
Han L, Yu K . Altered kinetics of nonhomologous end joining and class switch recombination in ligase IV-deficient B cells. J Exp Med 2008; 205: 2745–2753.
Rivera-Munoz P, Soulas-Sprauel P, Le Guyader G, Abramowski V, Bruneau S, Fischer A et al. Reduced immunoglobulin class switch recombination in the absence of Artemis. Blood 2009; 114: 3601–3609.
Soulas-Sprauel P, Le Guyader G, Rivera-Munoz P, Abramowski V, Olivier-Martin C, Goujet-Zalc C et al. Role for DNA repair factor XRCC4 in immunoglobulin class switch recombination. J Exp Med 2007; 204: 1717–1727.
Yan CT, Boboila C, Souza EK, Franco S, Hickernell TR, Murphy M et al. IgH class switching and translocations use a robust non-classical end-joining pathway. Nature 2007; 449: 478–482.
Helmink BA, Sleckman BP . The response to and repair of RAG-mediated DNA double-strand breaks. Annu Rev Immunol 2012; 30: 175–202.
Revy P, Buck D, le Deist F, de Villartay JP . The repair of DNA damages/modifications during the maturation of the immune system: lessons from human primary immunodeficiency disorders and animal models. Adv Immunol 2005; 87: 237–295.
Buck D, Moshous D, de Chasseval R, Ma Y, le Deist F, Cavazzana-Calvo M et al. Severe combined immunodeficiency and microcephaly in siblings with hypomorphic mutations in DNA ligase IV. Eur J Immunol 2006; 36: 224–235.
Enders A, Fisch P, Schwarz K, Duffner U, Pannicke U, Nikolopoulos E et al. A severe form of human combined immunodeficiency due to mutations in DNA ligase IV. J Immunol 2006; 176: 5060–5068.
O'Driscoll M, Cerosaletti KM, Girard PM, Dai Y, Stumm M, Kysela B et al. DNA ligase IV mutations identified in patients exhibiting developmental delay and immunodeficiency. Mol Cell 2001; 8: 1175–1185.
Buck D, Malivert L, de Chasseval R, Barraud A, Fondaneche MC, Sanal O et al. Cernunnos, a novel nonhomologous end-joining factor, is mutated in human immunodeficiency with microcephaly. Cell 2006; 124: 287–299.
Moshous D, Callebaut I, de Chasseval R, Corneo B, Cavazzana-Calvo M, Le Deist F et al. Artemis, a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell 2001; 105: 177–186.
Van der Burg M, Ijspeert H, Verkaik NS, Turul T, Wiegant WW, Morotomi-Yano K et al. A DNA-PKcs mutation in a radiosensitive T-B- SCID patient inhibits Artemis activation and nonhomologous end-joining. J Clin Invest 2009; 119: 91–98.
Woodbine L, Neal JA, Sasi NK, Shimada M, Deem K, Coleman H et al. PRKDC mutations in a SCID patient with profound neurological abnormalities. J Clin Invest 2013; 123: 2969–2980.
Woodbine L, Gennery AR, Jeggo PA . The clinical impact of deficiency in DNA non-homologous end-joining. DNA Repair (Amst) 2014; 16: 84–96.
O'Marcaigh AS, DeSantes K, Hu D, Pabst H, Horn B, Li L et al. Bone marrow transplantation for T-B- severe combined immunodeficiency disease in Athabascan-speaking native Americans. Bone Marrow Transplant 2001; 27: 703–709.
Antoine C, Muller S, Cant A, Cavazzana-Calvo M, Veys P, Vossen J et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968-99. Lancet 2003; 361: 553–560.
Dvorak CC, Cowan MJ . Radiosensitive severe combined immunodeficiency disease. Immunol Allergy Clin North Am 2010; 30: 125–142.
Filipovich A . Hematopoietic cell transplantation for correction of primary immunodeficiencies. Bone Marrow Transplant 2008; 42: S49–S52.
Ma Y, Pannicke U, Schwarz K, Lieber MR . Hairpin opening and overhang processing by an Artemis/DNA-dependent protein kinase complex in nonhomologous end joining and V(D)J recombination. Cell 2002; 108: 781–794.
Neven B, Leroy S, Decaluwe H, Le Deist F, Picard C, Moshous D et al. Long-term outcome after hematopoietic stem cell transplantation of a single-center cohort of 90 patients with severe combined immunodeficiency. Blood 2009; 113: 4114–4124.
Schuetz C, Neven B, Dvorak CC, Leroy S, Ege MJ, Pannicke U et al. SCID patients with ARTEMIS vs RAG deficiencies following HCT: increased risk of late toxicity in ARTEMIS-deficient SCID. Blood 2014; 123: 281–289.
Fischer A, Hacein-Bey-Abina S, Cavazzana-Calvo M . 20 years of gene therapy for SCID. Nat Immunol 2010; 11: 457–460.
Multhaup M, Karlen AD, Swanson DL, Wilber A, Somia NV, Cowan MJ et al. Cytotoxicity associated with artemis overexpression after lentiviral vector-mediated gene transfer. Hum Gene Ther 2010; 21: 865–875.
Sridharan DM, Whalen MK, Almendrala D, Cucinotta FA, Kawahara M, Yannone SM et al. Increased Artemis levels confer radioresistance to both high and low LET radiation exposures. Radiat Oncol 2012; 7: 96.
Ulus-Senguloglu G, Arlett CF, Plowman PN, Parnell J, Patel N, Bourton EC et al. Elevated expression of artemis in human fibroblast cells is associated with cellular radiosensitivity and increased apoptosis. Br J Cancer 2012; 107: 1506–1513.
Xiao C, Calado DP, Galler G, Thai TH, Patterson HC, Wang J et al. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell 2007; 131: 146–159.
Vera G, Rivera-Munoz P, Abramowski V, Malivert L, Lim A, Bole-Feysot C et al. Cernunnos deficiency reduces thymocyte life span and alters the T cell repertoire in mice and humans. Mol Cell Biol 2013; 33: 701–711.
Benjelloun F, Garrigue A, Demerens-de Chappedelaine C, Soulas-Sprauel P, Malassis-Seris M, Stockholm D et al. Stable and functional lymphoid reconstitution in artemis-deficient mice following lentiviral artemis gene transfer into hematopoietic stem cells. Mol Ther 2008; 16: 1490–1499.
Barreto V, Marques R, Demengeot J . Early death and severe lymphopenia caused by ubiquitous expression of the Rag1 and Rag2 genes in mice. Eur J Immunol 2001; 31: 3763–3772.
Wayne J, Suh H, Misulovin Z, Sokol KA, Inaba K, Nussenzweig MC . A regulatory role for recombinase activating genes, RAG-1 and RAG-2, in T cell development. Immunity 1994; 1: 95–107.
Wayne J, Suh H, Sokol KA, Petrie HT, Witmer-Pack M, Edelhoff S et al. TCR selection and allelic exclusion in RAG transgenic mice that exhibit abnormal T cell localization in lymph nodes and lymphatics. J Immunol 1994; 153: 5491–5502.
Oettinger MA, Schatz DG, Gorka C, Baltimore D . RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science 1990; 248: 1517–1523.
Schatz DG, Oettinger MA, Baltimore D . The V(D)J recombination activating gene, RAG-1. Cell 1989; 59: 1035–1048.
Difilippantonio MJ, Zhu J, Chen HT, Meffre E, Nussenzweig MC, Max EE et al. DNA repair protein Ku80 suppresses chromosomal aberrations and malignant transformation. Nature 2000; 404: 510–514.
Donehower LA . The p53-deficient mouse: a model for basic and applied cancer studies. Semin Cancer Biol 1996; 7: 269–278.
Frank KM, Sharpless NE, Gao Y, Sekiguchi JM, Ferguson DO, Zhu C et al. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol Cell 2000; 5: 993–1002.
Gao Y, Ferguson DO, Xie W, Manis JP, Sekiguchi J, Frank KM et al. Interplay of p53 and DNA-repair protein XRCC4 in tumorigenesis, genomic stability and development. Nature 2000; 404: 897–900.
Lim DS, Vogel H, Willerford DM, Sands AT, Platt KA, Hasty P . Analysis of ku80-mutant mice and cells with deficient levels of p53. Mol Cell Biol 2000; 20: 3772–3780.
Vanasse GJ, Halbrook J, Thomas S, Burgess A, Hoekstra MF, Disteche CM et al. Genetic pathway to recurrent chromosome translocations in murine lymphoma involves V(D)J recombinase. J Clin Invest 1999; 103: 1669–1675.
Li S, Lefranc MP, Miles JJ, Alamyar E, Giudicelli V, Duroux P et al. IMGT/HighV QUEST paradigm for T cell receptor IMGT clonotype diversity and next generation repertoire immunoprofiling. Nat Commun 2013; 4: 2333.
Acknowledgements
We thank Dr P Revy, Dr D Moshous, Dr I André-Schmutz and Dr C Lagresle-Peyrou for helpful discussion during the project. This work was supported by institutional grants from the Institut National de la Santé et de la Recherche Médicale (INSERM), the Ligue Nationale contre le Cancer (Equipe labellisée LA LIGUE), the Institut National du Cancer (INCa/IdF) and the European Research Council (ERC) (249816 PIDIMMUN). PR-M was supported by the Agence Nationale de la Recherche.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Rivera-Munoz, P., Abramowski, V., Jacquot, S. et al. Lymphopoiesis in transgenic mice over-expressing Artemis. Gene Ther 23, 176–186 (2016). https://doi.org/10.1038/gt.2015.95
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2015.95