Abstract
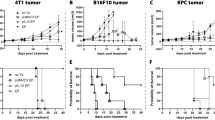
Given as a prophylactic treatment, a single muscle electrogene transfer of plasmid coding canstatin fused to human serum albumin (CanHSA), slowed down the development of two xenografted human carcinomas from mammary (MDA-MB-231) and prostate origin (PC-3) in nude mice and delayed lung metastatic spreading of B16F10 melanoma cells in syngenic mice. No effect was observed with unfused canstatin. The long lasting circulating blood level of CanHSA (20 ng ml−1) resulted in a profound disorganization of the tumor blood vessel network. However, when used as a curative treatment, on well-established tumors, CanHSA electrogenetherapy was ineffective in reducing tumor growth. As radiation is known to increase the αvβ3 and αvβ5 integrins, which are canstatin receptors, to extend the use of CanHSA electrogenetherapy, as a curative treatment, we explored the combination of CanHSA and ionizing radiation. We demonstrated a better efficacy (P=0.01) of the bitherapy over irradiation alone, as a result of strong vessel disorganization and dramatic increase of tumor cells apoptosis. This extremely simple virus free curative protocol could open the door to potential clinical applications, especially for prostate cancer that often develops radioresistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Folkman J . Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285: 1182–1186.
O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994; 79: 315–328.
O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997; 88: 277–285.
Hanahan D, Folkman J . Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86: 353–364.
Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003; 349: 427–434.
Cobleigh MA, Langmuir VK, Sledge GW, Miller KD, Haney L, Novotny WF et al. A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol 2003; 5: 117–124.
Ferrara N, Kerbel RS . Angiogenesis as a therapeutic target. Nature 2005; 438: 967–974.
Cooney MM, van Heeckeren W, Bhakta S, Ortiz J, Remick SC . Drug insight: vascular disrupting agents and angiogenesis--novel approaches for drug delivery. Nat Clin Pract Oncol 2006; 12: 682–692.
Maeshima Y, Manfredi M, Reimer C, Holthaus KA, Hopfer H, Chandamuri BR et al. Identification of the anti-angiogenic site within vascular basement membrane-derived tumstatin. J Biol Chem 2001; 276: 15240–15248.
Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K et al. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res 2000; 60: 2520–2526.
Kamphaus GD, Colorado PC, Panka DJ, Hopfer H, Ramchandran R, Torre A et al. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem 2000; 275: 1209–1215.
He GA, Luo JX, Zhang TY, Wang FY, Li RF . Canstatin-N fragment inhibits in vitro endothelial cell proliferation and suppresses in vivo tumor growth. Biochem Biophys Res Commun 2003; 312: 801–805.
Li YY, Qian GS, Huang GJ, Chen F, Qian P, Yu SC et al. Enhancement of antiangiogenic effects of human canstatin with a hypoxia-regulated transgene vector in lung cancer model. Cancer J 2006; 12: 136–146.
Griscelli F, Li H, Bennaceur-Griscelli A, Soria J, Opolon P, Soria C et al. Angiostatin gene transfer: inhibition of tumor growth in vivo by blockage of endothelial cell proliferation associated with a mitosis arrest. Proc Natl Acad Sci USA 1998; 95: 6367–6372.
Galaup A, Magnon C, Rouffiac V, Opolon P, Opolon D, Lassau N et al. Full kringles of plasminogen (aa 1–566) mediate complete regression of human MDA-MB-231 breast tumor xenografted in nude mice. Gene Therapy 2005; 10: 831–842.
Calvo A, Feldman AL, Libutti SK, Green JE . Adenovirus-mediated endostatin delivery results in inhibition of mammary gland tumor growth in C3(1)/SV40 T-antigen transgenic mice. Cancer Res 2002; 62: 3934–3938.
Magnon C, Galaup A, Mullan B, Rouffiac V, Bidart JM, Griscelli F et al. Canstatin acts on endothelial and tumor cells via mitochondrial damage initiated through interaction with alphavbeta3 and alphavbeta5 integrins. Cancer Res 2005; 65: 4353–4361.
Chen QR, Kumar D, Stass SA, Mixson AJ . Liposomes complexed to plasmids encoding angiostatin and endostatin inhibit breast cancer in nude mice. Cancer Res 1999; 59: 3308–3312.
Mir LM, Bureau MF, Rangara R, Schwartz B, Scherman D . Long-term, high level in vivo gene expression after electric pulse-mediated gene transfer into skeletal muscle. C R Acad Sci 1998; 321: 893–899.
Cichon T, Jamrozy L, Glogowska J, Missol-Kolka E, Szala S . Electrotransfer of gene encoding endostatin into normal and neoplastic mouse tissues: inhibition of primary tumor growth and metastatic spread. Cancer Gene Ther 2002; 9: 771–777.
Martel-Renoir D, Trochon-Joseph V, Galaup A, Bouquet C, Griscelli F, Opolon P et al. Coelectrotransfer to skeletal muscle of three plasmids coding for antiangiogenic factors and regulatory factors of the tetracycline-inducible system: tightly regulated expression, inhibition of transplanted tumor growth, and antimetastatic effect. Mol Ther 2003; 8: 425–433.
Trochon-Joseph V, Martel-Renoir D, Mir LM, Thomaidis A, Opolon P, Connault E et al. Evidence of antiangiogenic and antimetastatic activities of the recombinant disintegrin domain of metargidin. Cancer Res 2004; 64: 2062–2069.
Nakano A, Matsumori A, Kawamoto S, Tahara H, Yamato E, Sasayama S et al. Cytokine gene therapy for myocarditis by in vivo electroporation. Hum Gene Ther 2001; 12: 1289–1297.
Lucas ML, Heller L, Coppola D, Heller R . IL-12 plasmid delivery by in vivo electroporation for the successful treatment of established subcutaneous B16.F10 melanoma. Mol Ther 2002; 5: 668–675.
Nakamura H, Isaka Y, Tsujie M, Akagi Y, Sudo T, Ohno N et al. Electroporation-mediated PDGF receptor-IgG chimera gene transfer ameliorates experimental glomerulonephritis. Kidney Int 2001; 6: 2134–2145.
Chang Y, Prud'homme GJ . Intramuscular administration of expression plasmids encoding interferon-gamma receptor/IgG1 or IL-4/IgG1 chimeric proteins protects from autoimmunity. J Gene Med 1999; 1: 415–423.
Elie N, Plancoulaine B, Signolle JP, Herlin P . A simple way of quantifying immunostained cell nuclei on the whole histologic section. Cytometry A 2003; 56: 37–45.
Mauceri HJ, Hanna NN, Beckett MA, Gorski DH, Staba MJ, Stellato KA et al. Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature 1998; 394: 287–291.
Gorski DH, Mauceri HJ, Salloum RM, Gately S, Hellman S, Beckett MA et al. Potentiation of the antitumor effect of ionizing radiation by brief concomitant exposures to angiostatin. Cancer Res 1998; 58: 5686–5689.
Griscelli F, Li H, Cheong C, Opolon P, Bennaceur-Griscelli A, Vassal G et al. Combined effects of radiotherapy and angiostatin gene therapy in glioma tumor model. Proc Natl Acad Sci USA 2000; 97: 6698–6703.
Kaliberov SA, Buchsbaum DJ . Gene delivery and gene therapy of prostate cancer. Expert Opin Drug Deliv 2006; 1: 37–51.
Magnon C, Opolon P, Ricard M, Connault E, Ardouin P, Galaup A et al. Radiation and inhibition of angiogenesis by canstatin synergize to induce HIF-1alpha-mediated tumor apoptotic switch. J Clin Invest 2007; 117: 1844–1855.
Mehvar R, Shepard TL . Molecular-weight-dependent pharmacokinetics of fluorescein-labeled dextrans in rats. J Pharm Sci 1992; 81: 908–912.
Abdollahi A, Griggs DW, Zieher H, Roth A, Lipson KE, Saffrich R et al. Inhibition of alpha(v)beta3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin Cancer Res 2005; 1: 6270–6279.
Cordes N . Integrin-mediated cell-matrix interactions for prosurvival and antiapoptotic signaling after genotoxic injury. Cancer Lett 2006; 242: 11–29.
Lee CG, Heijn M, di Tomasco E, Griffon-Etienne G, Ancukiewicz M, Koike C et al. Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res 2000; 60: 5565–5570.
Salloum RM, Jaskowiak NT, Mauceri HJ, Seetharam S, Beckett MA, Koons AM et al. NM-3, an isocoumarin, increases the antitumor effects of radiotherapy without toxicity. Cancer Res 2000; 60: 6958–6963.
Geng L, Donnely E, McMahon G, Lin C, Sierra-Rivera E, Oshinka H et al. Inhibition of vascular endothelial growth factor receptor signaling leads to reversal of tumor resistance to radiotherapy. Cancer Res 2001; 61: 2413–2419.
Griffin RJ, Williams BW, Wild R, Cherrington J, Park H, Song CW . Silmutaneous inhibition of the receptor kinase activity of vascular endothelial, fibroblast, and platelet-derived growth factors suppresses tumor growth and enhances tumor radiation response. Cancer Res 2002; 62: 1702–1706.
Citrin D, Menard C, Camphausen K . Combining radiotherapy and angiogenesis inhibitors: clinical trial design. Int J Radiat Oncol Biol Phys 2006; 64: 15–25.
O'Reilly MS . Radiation combined with antiangiogenic and antivascular agents. Semin Radiat Oncol 2006; 1: 45–50.
Isaacs WB, Carter BS, Ewing CM . Wild-type p53 suppresses growth of human prostate cancer cells containing mutant p53 alleles. Cancer Res 991; 51: 4716–4720.
Cowden Dahl KD, Robertson SE, Weaver VM, Simon MC . Hypoxia-inducible factor regulates alphavbeta3 integrin cell surface expression. Mol Biol Cell 2005; 4: 1901–1912.
Albert JM, Cao C, Geng L, Leavitt L, Hallahan DE, Lu B . Integrin alpha v beta 3 antagonist Cilengitide enhances efficacy of radiotherapy in endothelial cell and non-small-cell lung cancer models. Int J Radiat Oncol Biol Phys 2006; 65: 1536–1543.
Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998; 394: 485–490.
Harris AL . Hypoxia-a key regulatory factor in tumour growth. Nat Rev Cancer 2002; 1: 38–47.
Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003; 300: 1155–1159.
Moeller BJ, Cao Y, Li CY, Dewhirst MW . Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 2004; 5: 429–441.
Acknowledgements
This paper was devoted to the memory of Patrice Ardouin, associate director of animal facility at Institut Gustave Roussy. We sincerely thank Monique Stanciu and Annie Rouches for excellent technical assistance and animal care. This work was supported by the Centre National de la Recherche Scientifique, the Institut Gustave Roussy, l'ARC Association de Recherche contre le Cancer, l'EDF (electricité de France) and University Paris XI. Claire Magnon was supported by Assistance Publique-Hôpitaux de Paris, the Institut National de la Santé et de la Recherche Médicale and Commissariat à l'Energie Atomique.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Magnon, C., Opolon, P., Connault, E. et al. Canstatin gene electrotransfer combined with radiotherapy: preclinical trials for cancer treatment. Gene Ther 15, 1436–1445 (2008). https://doi.org/10.1038/gt.2008.100
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2008.100