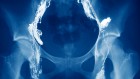
Richard Nixon signed the National Cancer Act at the White House on 23 December 1971. Since then, the idea of an ‘all-out assault’ on cancer has been moderated, with progress understood to be more likely to occur in small steps.Credit: Linda Bartlett/NIH/National Cancer Institute
In 1970, advisers to the US Senate attempted to forecast the evolution of cancer treatment. “The long-term future may belong to the immunologist and the geneticist, the intermediate future to the chemotherapist,” they wrote, “but the present and the immediate future belong in the main to the surgeon and to some extent to the radiologist.” More than 50 years later — and well into the ‘long-term future’ of genetics and immunology — their predictions have proved remarkably prescient.
The advisers had been called in to consult on what would become the National Cancer Act of 1971, the opening salvo of then-president Richard Nixon’s “war on cancer”. Cancer was the second leading cause of death in the United States, and the act — passed in December 1971 — called for US$1.5 billion to be poured into cancer research over three years, equivalent to more than $10 billion today.
The act strengthened the nation’s cancer-research infrastructure, creating a national clinical-trials network, as well as a host of specialized cancer research centres. But, from the start, Nixon’s focus on finding a swift cure for cancer was criticized as naive. Some researchers cautioned that cancer is a complex disease, and that progress was likely to be made in small, hard-won steps rather than a single, decisive cure. That prediction also turned out to be correct.
Half a century after the National Cancer Act became law, cancer is still the second leading cause of death in the United States. The act has fostered tremendous advances, in large part through a greater understanding of the biology that underlies the disease. But cancer research still faces challenges, not all of them scientific. Many therapies are too expensive for individuals or health-care systems: in the United States, 42% of people with cancer experienced severe financial hardship within two years of diagnosis, and, in many countries, innovative cancer therapies such as immunotherapies are out of reach for the majority.
The era of massive cancer sequencing projects has reached a turning point
In 1971, there were already signposts indicating the directions in which cancer research could lead. It was the year in which researchers published results of the first clinical trial of tamoxifen, a hormonal therapy for breast cancer. The US Food and Drug Administration (FDA) approved the drug, in 1978, to treat advanced cancers, and tamoxifen has since become a mainstay of breast cancer therapy.
When the act was passed, researchers already knew that the genomes of cancerous cells were chaotic and unstable. By 1976, cancer cytogeneticist Peter Nowell had realized that such genetic variability would make the task of finding therapies that much harder and more complex. Today, we know that cancer is not one but many different diseases. The complexities are mind-boggling — which is one of the reasons why cancer is so difficult to cure. Large-scale tumour-sequencing projects have taught us more about how each cancer type can be broken down into subtypes of subtypes, and that each individual tumour has a unique molecular make-up. Furthermore, this molecular signature shifts as the cancer progresses and responds to treatment.
Over the past 50 years, scientists have made remarkable progress in understanding this complexity through projects carried out by basic and clinical researchers working ever-more closely. The Cancer Genome Atlas and Pan-Cancer Analysis of Whole Genomes projects have revealed both the complexity of cancer and important clues to new treatments. Discoveries made during basic research on the cell cycle have led to the development of anticancer drugs that target certain cell-cycle proteins.
Last year, the FDA approved a drug for some non-small cell lung cancers that disables a mutant form of the KRAS protein. The KRAS gene is closely related to the first human oncogene — a gene with the potential to cause cancer — to be discovered, a decade or so after the act was passed. And therapies that target the immune system have opened up new avenues of treatment for several cancers.
COVID, racism, China: three tests for the next NIH leader
Such progress has saved lives. In 1971, only half of people in the United States diagnosed with cancer would live for more than five years beyond that diagnosis; now the fraction is around two-thirds. Scientists have since recognized the value of early detection, and in many countries there are early-detection programmes for some of the most common cancers, including breast and colon cancers.
The advances fuelled by the National Cancer Act have also spilt over into other fields. The study of how cancer cells interact with one another in tumours has fuelled a broader understanding of cell–cell interactions. Synthetic immune cells designed to fight cancer are now being studied as potential treatments for conditions including fibrosis of the heart and lungs. And large clinical trials designed to test multiple interventions and to adapt to interim data — a novel study design pioneered in cancer research — have been pivotal to the discovery of ways to treat COVID-19.
Today, scientists rarely talk of a broad cure for cancer. Instead, as Norman Sharpless, the director of the US National Cancer Institute, says, the aim is to end cancer “as we know it”. Some cancer might be an inevitable consequence of ageing, he says, but researchers can make headway against the shortcomings of current cancer therapies, by, for example, tackling the cancers for which there is little to offer patients; finding ways to reduce the side effects of harsh cancer therapies; and addressing the racial and socio-economic inequalities that affect access to clinical trials and treatments. The bulk of the data that have been painstakingly gathered over the past half-century were collected from white people — a bias that must be corrected.
The combination of technological advances with continued collaboration between basic and clinical researchers could sustain the momentum generated by the act into the next 50 years — and take the field ever further from the days when surgery and radiotherapy were the only treatments.

 The era of massive cancer sequencing projects has reached a turning point
The era of massive cancer sequencing projects has reached a turning point
 Replicating scientific results is tough — but essential
Replicating scientific results is tough — but essential
 COVID, racism, China: three tests for the next NIH leader
COVID, racism, China: three tests for the next NIH leader