Abstract
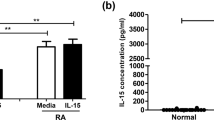
Rheumatoid arthritis is an autoimmune disease that primarily affects the limbs, but the pathogenic mechanism remains unclear. γδ T cells, a T-cell subpopulation, are characterized by multiple biological functions and associated with a variety of diseases. This study investigated the antigen-presenting effects of γδ T cells and their relationship with rheumatoid arthritis development. We found that Vγ9Vδ2 T cells (the predominant subtype of γδ T cells in peripheral blood) were activated by isopentenyl pyrophosphate to continuously proliferate and differentiate into effector memory cells. The effector memory Vγ9Vδ2 T cells exhibited phenotypic characteristics of specific antigen-presenting cells, including high HLA-DR and CD80/86 expression. These Vγ9Vδ2 T cells could present soluble antigens and synthetic peptides to CD4+ T cells. Vγ9Vδ2 T cells with different phenotypes showed different cytokine secretion patterns. Effector memory Vγ9Vδ2 T cells simultaneously secreted not only interferon (IFN)-γ but also IL-17. The peripheral blood and joint synovial fluid from RA patients contained numerous heterogeneous γδ T cells that were predominantly effector memory Vγ9Vδ2 T cells with the ability to secrete inflammatory factors. We also found that γδ T cells had a similar antigen-presenting capability to B cells. These results suggest that during the development of rheumatoid arthritis, γδ T cells can aggravate immune dysfunction and produce abnormal immune damage by secreting cytokines and inducing inflammatory cells to participate in synergistic inflammatory responses. Furthermore, γδ T cells can behave similarly to B cells to present viral peptides and autoantigen peptides to CD4+ T cells, thus sustaining CD4+ T-cell activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Takeda T, Mizugaki Y, Matsubara L, Imai S, Koike T, Takada K . Lytic Epstein–Barr virus infection in the synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum 2000; 43: 1218–1225.
Zoschke D, Segall M . Dw subtypes of DR4 in rheumatoid arthritis: evidence for a preferential association with Dw4. Hum Immunol 1986; 15: 118–124.
Nepom GT, Byers P, Seyfried C, Healey LA, Wilske KR, Stage D et al. HLA genes associated with rheumatoid arthritis. Identification of susceptibility alleles using specific oligonucleotide probes. Arthritis Rheum 1989; 32: 15–21.
Roudier J, Petersen J, Rhodes GH, Luka J, Carson DA . Susceptibility to rheumatoid arthritis maps to a T-cell epitope shared by the HLA-Dw4 DR beta-1 chain and the Epstein–Barr virus glycoprotein gp110. Proc Natl Acad Sci USA 1989; 86: 5104–5108.
Benoist C, Mathis D . Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry? Nat Immunol 2001; 2: 797–801.
Cope AP . T cells in rheumatoid arthritis. Arthritis Res Ther 2008; 10: S1–S10.
Cope AP, Schulze-Koops H, Aringer M . The central role of T cells in rheumatoid arthritis. Clin Exp Rheumatol 2007; 25: S4–S11.
Thomas R, Turner M, Cope AP . High avidity autoreactive T cells with a low signalling capacity through the T-cell receptor: central to rheumatoid arthritis pathogenesis? Arthritis Res Ther 2008; 10: 210.
Tran CN, Lundy SK, Fox DA . Synovial biology and T cells in rheumatoid arthritis. Pathophysiology 2005; 12: 183–189.
Panayi GS . B cells: a fundamental role in the pathogenesis of rheumatoid arthritis? Rheumatology (Oxford) 2005; 44: ii3–ii7.
Bodman-Smith MD, Anand A, Durand V, Youinou PY, Lydyard PM . Decreased expression of FcgammaRIII (CD16) by gammadelta T cells in patients with rheumatoid arthritis. Immunology 2000; 99: 498–503.
Holoshitz J . Activation of gammadelta T cells by mycobacterial antigens in rheumatoid arthritis. Microbes Infect 1999; 1: 197–202.
Roark CL, Simonian PL, Fontenot AP, Born WK, O'Brien RL . gammadelta T cells: an important source of IL-17. Curr Opin Immunol 2008; 20: 353–357.
Umemura M, Kawabe T, Shudo K, Kidoya H, Fukui M, Asano M et al. Involvement of IL-17 in Fas ligand-induced inflammation. Int Immunol 2004; 16: 1099–1108.
Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette–Guerin infection. J Immunol 2007; 178: 3786–3796.
Fischer S, Scheffler A, Kabelitz D . Activation of human γδ T-cells by heat-treated mistletoe plant extracts. Immunol Lett 1996; 52: 69–72.
Hayday AC . γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol 2000; 18: 975–1026.
Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C et al. Differentiation of effector/memory Vδ2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med 2003; 198: 391–397.
Ferlazzo V, Sferrazza C, Caccamo N, Di Fede G, Di Lorenzo G, D'Asaro M et al. In vitro effects of aminobisphosphonates on Vγ9Vδ2 T cell activation and differentiation. Int J Immunopathol Pharmacol 2006; 19: 309–317
Born WK, Reardon CL, O'Brien RL . The function of gammadelta T cells in innate immunity. Curr Opin Immunol 2006; 18: 31–38.
Beetz S, Wesch D, Marischen L, Welte S, Oberg HH, Kabelitz D . Innate immune functions of human gammadelta T cells. Immunobiology 2008; 213: 173–182.
Gryglewski A, Majcher P, Bryniarski K, Konturek S, Ptak M, Ptak W et al. Mesenteric lymph node Tgammadelta cells induced by gastrectomy in mice suppress cell-mediated immune response in vitro via released TGF-beta. J Surg Res 2007; 142: 66–71.
Kabelitz D, Wesch D, He W . Perspectives of gammadelta T cells in tumor immunology. Cancer Res 2007; 67: 5–8.
Green AE, Lissina A, Hutchinson SL, Hewitt RE, Temple B, James D et al. Recognition of nonpeptide antigens by human Vγ9Vδ2 T cells requires contact with cells of human origin. Clin Exp Immunol 2004; 136: 472–482.
Kabelitz D, Glatzel A, Wesch D . Antigen recognition by human gammadelta T lymphocytes. Int Arch Allergy Immunol 2000; 122: 1–7.
Peng MY, Wang ZH, Yao CY, Jiang LN, Jin QL, Wang J et al. Interleukin 17-producing gamma delta T cells increased in patients with active pulmonary tuberculosis. Cell Mol Immunol 2008; 5: 203–208.
Chen Y, Lu H, Li B, Wang W, Zhang H . Stimulatory effect of purified mycobacterium tuberculosis peptide antigen on human γδT lymphocyte proliferation. Chin J Immunol 2004; 20: 661–664.
Ito Y, Usui T, Kobayashi S, Iguchi-Hashimoto M, Ito H, Yoshitomi H et al. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum 2009; 60: 2294–2303.
Ness-Schwickerath KJ, Jin C, Morita CT . Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells. J Immunol 2010; 184: 7268–7280.
Pöllinger B, Junt T, Metzler B, Walker UA, Tyndall A, Allard C et al. Th17 cells, not IL-17+ gamma/delta T cells, drive arthritic bone destruction in mice and humans. J Immunol 2011; 186: 2602–2612.
Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S et al. Gamma/delta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity 2010; 33: 351–363.
Silva-Santos B . gamma/delta cells making IL-17. Blood 2011; 118: 3–5.
Moser B, Eberl M . gammadelta T cells: novel initiators of adaptive immunity. Immunol Rev 2007; 215: 89–102.
Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG . BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity 2000; 12: 471–481.
Brandes M, Willimann K, Lang AB, Nam KH, Jin C, Brenner MB et al. Flexible migration program regulates gamma delta T-cell involvement in humoral immunity. Blood 2003; 102: 3693–3701.
Acknowledgements
This work was supported by the grants from the following: National Natural Science Foundation of China (no. 30471593, 30872304 and 81072470), Shanghai Commission of Science and Technology (no. 10JC14 08500 and 10ZR1426100), Shanghai Leading Academic Discipline-Surgery (no. S30204-K01), Shanghai Municipal education Commission (no. J50207 and 09YZ102), Shanghai Institute of Immunology (no. 08-A04), Clinical Medicine Technology Development Foundation of Jiangsu University (no. JLY2010091) and Foundation of Shanghai Xuhui Central Hospital (no. 2011XHCH07).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hu, C., Qian, L., Miao, Y. et al. Antigen-presenting effects of effector memory Vγ9Vδ2 T cells in rheumatoid arthritis. Cell Mol Immunol 9, 245–254 (2012). https://doi.org/10.1038/cmi.2011.50
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2011.50
Keywords
This article is cited by
-
Dominance and improved survivability of human γδT17 cell subset aggravates the immunopathogenesis of pemphigus vulgaris
Immunologic Research (2024)
-
Immune-mediated syndromes following intravenous bisphosphonate therapy
Inflammopharmacology (2017)
-
Reduced pro-inflammatory profile of γδT cells in pregnant patients with rheumatoid arthritis
Arthritis Research & Therapy (2016)
-
Identification and characterization of latency-associated peptide-expressing γδ T cells
Nature Communications (2015)
-
Human Vγ9Vδ2-T cells efficiently kill influenza virus-infected lung alveolar epithelial cells
Cellular & Molecular Immunology (2013)