Abstract
Novel therapeutic targets are required to protect the heart against cell death from acute ischemia–reperfusion injury (IRI). Mutations in the DJ-1 (PARK7) gene in dopaminergic neurons induce mitochondrial dysfunction and a genetic form of Parkinson’s disease. Genetic ablation of DJ-1 renders the brain more susceptible to cell death following ischemia–reperfusion in a model of stroke. Although DJ-1 is present in the heart, its role there is currently unclear. We sought to investigate whether mitochondrial DJ-1 may protect the heart against cell death from acute IRI by preventing mitochondrial dysfunction. Overexpression of DJ-1 in HL-1 cardiac cells conferred the following beneficial effects: reduced cell death following simulated IRI (30.4±4.7% with DJ-1 versus 52.9±4.7% in control; n=5, P<0.05); delayed mitochondrial permeability transition pore (MPTP) opening (a critical mediator of cell death) (260±33 s with DJ-1 versus 121±12 s in control; n=6, P<0.05); and induction of mitochondrial elongation (81.3±2.5% with DJ-1 versus 62.0±2.8% in control; n=6 cells, P<0.05). These beneficial effects of DJ-1 were absent in cells expressing the non-functional DJ-1L166P and DJ-1Cys106A mutants. Adult mice devoid of DJ-1 (KO) were found to be more susceptible to cell death from in vivo IRI with larger myocardial infarct sizes (50.9±3.5% DJ-1 KO versus 41.1±2.5% in DJ-1 WT; n≥7, P<0.05) and resistant to cardioprotection by ischemic preconditioning. DJ-1 KO hearts showed increased mitochondrial fragmentation on electron microscopy, although there were no differences in calcium-induced MPTP opening, mitochondrial respiratory function or myocardial ATP levels. We demonstrate that loss of DJ-1 protects the heart from acute IRI cell death by preventing mitochondrial dysfunction. We propose that DJ-1 may represent a novel therapeutic target for cardioprotection.
Similar content being viewed by others
Main
Novel therapeutic strategies capable of protecting the heart against acute ischemia–reperfusion injury (IRI) are needed to reduce myocardial infarct (MI) size, preserve left ventricular (LV) systolic function and improve clinical outcomes in patients with ischemic heart disease (IHD), the leading cause of death and disability worldwide. Mitochondrial dysfunction induced by acute IRI is a major determinant of myocardial injury and cardiomyocyte cell death. Preservation of cardiomyocyte mitochondrial function in patients following IRI may therefore be expected to reduce cell death, prevent the onset of heart failure and ultimately improve long-term prognosis of IHD patients.
DJ-1 (also known as PARK7 (Parkinson disease (autosomal recessive, early onset) 7)) is a widely expressed and conserved mitochondrial protein that has been implicated in numerous pathologies, most notably in neurodegeneration where mutations in DJ-1 (e.g., the L166P mutation) ultimately result in an early-onset familial form of Parkinson’s disease.1 Several experimental studies have elucidated important properties for DJ-1, including oxidative stress sensing, reactive oxygen species scavenging2, 3, 4 and preservation of normal mitochondrial function in the brain.2, 5, 6 Given its crucial role in maintaining mitochondrial function, DJ-1 represents a potential target for novel therapies for other pathologies involving mitochondrial dysfunction and cell death, including IRI. Indeed, animal studies have shown that DJ-1 ablation renders the brain more susceptible to cell death in the setting of stroke.7 Although DJ-1 is expressed in the heart,1 its role there is not well understood.
In the current study, we investigated the role of mitochondrial DJ-1 in the heart and its potential as a novel therapeutic target for protecting the heart against cell death following acute IRI.
Results
DJ-1 overexpression reduced HL-1 cell death from simulated IRI
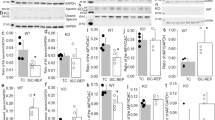
Cell death was assessed in the HL-1 cardiac cell line in response to simulated IRI. WT DJ-1 overexpressing cells exhibited significantly lower cell death following simulated IRI (Figure 1a: cell death: WT DJ-1 30.4± 4.7% versus vector control 52.9±4.7%, n=5, *P<0.05) to a similar extent as the insulin-positive control. Expression of mitochondrial targeted DJ-1 (MitoDJ-1) also reduced cell death, although this was not statistically significant (Figure 1a: cell death: MitoDJ-1 38.5±3.5% versus vector control 52.9±4.7%, n=5, NSP>0.05 (NS, not statistically significant)). Interestingly, expression of mutant DJ-1 did not affect cell death following simulated IRI (Figure 1a: cell death: L166P 55.1±3.2%, Cys106A 62.8±7.3% versus vector control 52.9±4.7%; n=5, NSP>0.05). Cell death in un-transfected time-matched normoxic control cells was <5.0%, and this was not significantly altered by transgene expression (data not shown).
Assessment of the role of DJ-1 in vitro. Modulation of DJ-1 protein in vitro in HL-1 cells by transfection with WT and MitoDJ-1 and non-functional mutant DJ-1 (L166P and Cys106A). (a) Cell survival in response to simulated IRI, n=5/group. (b) MPTP opening in response to laser-induced oxidative stress, n≥5/group. (c) Representative images of TMRM-loaded mitochondria: (i) Control, (ii) WT-DJ (more elongated mitochondria), (iii) Cys106A and (iv) MitoDJ-1 (more elongated mitochondria). (d) Mitochondrial morphology, n≥5/group. Significance assessed by one-way ANOVA; significance indicated against comparison with control (white bars). *P<0.05, **P<0.01
DJ-1 overexpression delayed mitochondrial permeability transition pore (MPTP) opening in HL-1 cells
MPTP opening is a critical mediator of cell death. DJ-1 overexpression significantly delayed MPTP opening in HL-1 cells (Figure 1b: normalized half-times: WT DJ-1 260±33 s versus vector control 121±12 s, n=6, *P<0.05). Expression of MitoDJ-1 also significantly delayed MPTP opening (Figure 1b: normalized half-times: MitoDJ-1 290±42 s versus vector control 121±12 s, n≥5, **P<0.01). Expression of DJ-1 mutants, L166P and Cys106A did not significantly affect MPTP opening times in this model (Figure 1b: normalized half-times: L166P 211±25 s, Cys106A 190±33 s versus vector control 121±12 s, n=6, NSP>0.05).
DJ-1 overexpression induced mitochondrial elongation in HL-1 cells
Mitochondrial morphology is an important indicator of cellular health and susceptibility to cell death; where mitochondrial elongation confers a cardioprotective phenotype. DJ-1 overexpression increased the proportion of cells displaying predominantly elongated mitochondria (Figure 1d: cells with elongated mitochondria: WT DJ-1 81.3±2.5% versus vector control 62.0± 2.8%; n=6, *P<0.05). Expression of MitoDJ-1 also induced significant mitochondrial elongation (Figure 1d: cells with elongated mitochondria: MitoDJ-1 83.2±7.1% versus vector control 62.0± 2.8%; n≥5, *P<0.05). Expression of DJ-1 mutants did not significantly affect mitochondrial morphology (Figure 1d: cells with elongated mitochondria: L166P 67.9±5.7%, Cys106A 56.4±4.5% versus vector control 62.0± 2.8%; n=6, NSP>0.05).
Cardiac phenotype in DJ-1 WT and knockout (KO) mice
There were no significant differences in anterior and posterior LV wall dimensions during systole or diastole (examples of echocardiographic images are provided in Supplementary Figure S1). Isoproterenol stress yielded a significant increase in heart rate in both DJ-1 WT and KO animals (Figure 2a: heart rate in beats per minute: WT DJ-1 baseline 498.5±19.9 and isoproterenol 620.2±10.2, n=6, P<0.05; DJ-1 KO baseline 505±25.9 and isoproterenol 628.8±17.0, n=5, P<0.05) and fractional shortening (Figure 2b: percentage of fractional shortening: WT DJ-1 baseline 34.7±3.1 and isoproterenol 63.0±1.2, n=6, P<0.05; DJ-1 KO baseline 41.2±4.1 and isoproterenol 65.4±3.2, n=5, P<0.05). There were no differences in cardiac output or stroke volume between DJ-1 WT and KO mice at baseline or following isoproterenol challenge (Figures 2c and d).
Echocardiographic phenotyping of DJ-1 WT and KO animals. (a–d) Isoproterenol (4 ng/g) treatment significantly increased the heart rate and fractional shortening in both DJ-1 WT and KO mice (n≥5/group; significance assessed by one-way ANOVA comparing WT Basal with WT ISO and KO Basal with KO ISO, where **P<0.01 and ***P<0.001). There were no significant differences in any cardiac parameter between DJ-1 WT and KO mice at baseline or in response to isoproterenol challenge (n≥5/group, significance assessed by one-way ANOVA comparing WT and KO, NSP>0.05)
DJ-1 KO increased susceptibility to cell death from IRI in vivo
DJ-1 KO mice exhibited a significant increase in cardiomyocyte cell death following in vivo IRI compared with DJ-1 WT ones (Figure 3a: infarct size %IS/AAR (infarct size/area-at-risk): DJ-1 KO 50.9±3.5% versus DJ-1 WT 41.1±2.5; n≥7/group, *P<0.05). Interestingly, DJ-1 KO mice appeared to be partially resistant to the MI size limiting effects of ischemic preconditioning (IPC) (Figures 3b and c: %IS/AAR in DJ-1 KO mice: control 50.9±3.5% versus IPC 39.4±4.1%, n=8/group, NSP>0.05). The extent of cardioprotection afforded by IPC was ≈47% in WTs compared with only ≈23% in KOs. There were no significant differences in body weight, heart weight or area-at-risk between any treatment groups in these experiments.
Susceptibility of DJ-1 KO to in vivo IRI. Infarct size following in vivo IRI in DJ-1 WT and KO mice. IS normalized to myocardial AAR to give IS/AAR%. (a) IS/AAR% in DJ-1 WT and KO mice subjected to standard IRI model of 45 min ischemia and 24 h reperfusion, n≥7/group. (b and c) IS/AAR% where standard IPC protocol of 5 min ischemia and 5 min reperfusion applied before main ischemic insult, n≥7/group. Significance assessed by unpaired t-test, *P<0.05, ***P<0.001. No statistical significance indicated as NSP>0.05
Calcium-induced MPTP opening in DJ-1 WT and DJ-1 KO mice
It was not possible to examine MPTP opening in primary isolated cardiomyocytes, as there was a strong trend to decreased mitochondrial membrane potential in DJ-1 KO hearts (Supplementary Figure S2), thereby precluding the use of the tetramethyl rhodamine methyl ester (TMRM)-based MPTP opening model. There were no differences in the swelling of mitochondria isolated from DJ-1 WT or DJ-1 KO hearts, suggesting that there was no difference in MPTP opening susceptibility (Figures 4a–c). As expected, ciclosporin A (CsA) treatment prevented calcium-induced mitochondrial swelling in both DJ-1 WT and DJ-1 KO mitochondria (Figure 4d).
Calcium-induced mitochondrial swelling in isolated mitochondria. Mitochondria isolated from DJ-1 WT and KO hearts were subjected to calcium-induced mitochondrial swelling. Extent of mitochondrial swelling was assessed by optical density using spectrophotometer. (a) Baseline. (b) Calcium chloride (CaCl2) 600 μM. (c) CaCl2 800 μM. (d) CsA pre-treatment at 1 μM for 15 min before swelling assay using 600 μM CaCl2. No apparent differences in mitochondrial swelling between DJ-1 WT and KO mitochondria, n=6/group
DJ-1 KO hearts display increased mitochondrial fragmentation
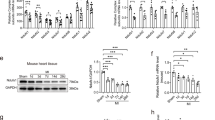
At baseline, DJ-1 KO hearts exhibited a significantly greater proportion of short mitochondria (<1 sarcomere in length) and a concurrent decrease in longer mitochondria (≥1 sarcomere in length) (Figures 5a and b, n≥3/group). Sublethal ischemia resulted in increased proportions of short mitochondria in both DJ-1 WT and KO hearts. Interestingly, following ischemia, there was no significant difference in mitochondrial length between DJ-1 WT and DJ-1 KO hearts (see Supplementary Figure S3).
Mitochondrial morphology in DJ-1 WT and KO hearts at baseline. Mitochondrial morphology in DJ-1 WT and KO hearts was assessed using EM of hearts isolated at baseline. (a) Representative EM images showing mitochondrial morphology in DJ-1 WT (i) and KO (ii) hearts at baseline; scale bar=2 μM. (b) Assessment of mitochondrial morphology by quantification of the proportion of mitochondria<1 sarcomere in length, equivalent to 1 sarcomere in length and >1 sarcomere in length, n≥3/group. Significance assessed by one-way ANOVA; significance indicated against comparison with DJ-1 WT control (white bars), *P<0.05, **P<0.01, ***P<0.001
Myocardial adenosine triphosphate (ATP) levels in DJ-1 WT and DJ-1 KO mice
There were no significant differences in myocardial ATP levels between DJ-1 WT and DJ-1 KO mice at baseline (Figure 6a, n≥5/group, NSP>0.05).
Functional characterization of DJ-1 WT and KO hearts. (a) Myocardial ATP assay in DJ-1 WT and KO hearts. ATP assessed using luminescence assay and normalized to DJ-1 WT baseline, n≥5/group. No significant difference in ATP levels between DJ-1 WT and KOs; significance assessed by unpaired t-test, NSP>0.05. (b) Mitochondrial respiration assessed by oxygen flux readings for individual respiratory complexes in mitochondria isolated from DJ-1 WT and KO hearts, n≥5/group. No significant difference in mitochondrial respiratory function; significance assessed by one-way ANOVA, significance indicated against comparison with DJ-1 WT control (white bars), NSP>0.05. Characterization of ATP levels and mitochondrial respiration in DJ-1 WT hearts is shown in Supplementary Figure S4
Mitochondrial respiration in DJ-1 WT and DJ-1 KO mice
There were no differences in mitochondrial respiratory rates between the DJ-1 WT and DJ-1 KO hearts at baseline (Figure 6b, n≥5/group, NSP>0.05).
The effect of DJ-1 genetic ablation on myocardial ATP levels and mitochondrial respiration could not be examined effectively in these animals, as a sublethal ischemic insult (of 20 min ischemia) did not induce a significant effect (see Supplementary Figure S4). The effect of an extended ischemic insult was not feasible given the clear effect of DJ-1 genetic ablation on cell survival indicated here.
Discussion
In the present study, we demonstrate that mitochondrial DJ-1 may be an important therapeutic target for cardioprotection as evidenced by the following:(1) overexpression of WT DJ-1 but not its mutant forms reduced cell death following simulated IRI, delayed MPTP opening, and induced mitochondrial elongation in the HL-1 cardiac cell line; and (2) adult murine hearts deficient in DJ-1 were found to be more susceptible to cell death from IRI as evidenced by a larger MI size, a finding which was associated with increased mitochondrial fragmentation.
The beneficial effects of WT DJ-1 overexpression in HL-1 cells observed in our study appeared to be dependent on the ability of DJ-1 to dimerize and sense oxidation, as these effects were absent with the mutant forms of the protein, DJ-1L166P (which is unable to dimerize) and DJ-1Cys106A (in which Cys106 is resistant to oxidation). Dimerization and oxidation of DJ-1 are both known to be critical steps in its activation and mitochondrial translocation.8, 9 The beneficial effects of DJ-1 overexpression included reduced cell death from simulated IRI and protective effects on mitochondrial function, including delayed onset of MPTP opening (a critical mediator of cell death)10 and mitochondrial elongation (a change in mitochondrial morphology which has been reported to confer a cardioprotective phenotype).11 Although it should be noted that the cell death assay performed here could be improved by use of a more quantitative end point such as flow cytometry, the protective effect of DJ-1 overexpression against simulated IRI is also confirmed by the recent demonstration of a similar effect in H9c2 rat myoblasts.12
Adult mice deficient in DJ-1 were found to display no overt cardiac phenotype in terms of cardiac dimensions and function. This would suggest that DJ-1 does not appear to have a critical role in cardiac function under physiological conditions. The lack of any obvious cardiac phenotype such as LV hypertrophy therefore allowed us to examine the effect of DJ-1 deficiency on susceptibility to IRI, without any confounding myocardial pathology. Crucially, we found that hearts deficient in DJ-1 were more susceptible to cell death from acute IRI, as evidenced by a larger MI size. This finding suggests that endogenous DJ-1 may be an important target for cardioprotection. Interestingly, we also found that adult hearts lacking DJ-1 were resistant to the endogenous cardioprotective phenomenon of IPC, suggesting that DJ-1 may contribute to the signaling pathway underlying IPC. DJ-1 has also been reported to act as a mediator of hypoxic preconditioning in H9c2 rat myoblasts.13 In the brain, it has been reported that DJ-1 can increase the activity of the pro-survival kinase, Akt.13, 14, 15 Whether DJ-1 is required for the activation of Akt in the setting of IPC is an interesting possibility that remains to be explored.
Because DJ-1 overexpression in the HL-1 cell line delayed the onset of MPTP opening, we were expecting to observe an increased susceptibility to MPTP opening in cardiac mitochondria isolated from DJ-1 KO mice, but this was not the case. The reason for this discrepancy may relate to the experimental model used to induce and assess MPTP opening. In the HL-1 cell studies, MPTP opening was induced by oxidative stress in intact cardiomyocytes, whereas in the DJ-1 KO mice, MPTP opening was induced by calcium using isolated mitochondria. It was not possible to use the oxidative stress TMRM model of MPTP opening in cardiomyocytes isolated from DJ-1 KO hearts given the prominent trend to decreased mitochondrial membrane potential at baseline in DJ-1 KO cardiomyocytes compared with wild-type (WT) controls (Supplementary Figure S2). Many of the beneficial effects of DJ-1 in the brain relate to its anti-oxidant properties, and its ability to translocate to the mitochondria, and this may in part explain why we did not see the expected effects of DJ-1 ablation on MPTP opening in the isolated mitochondria model of calcium-induced MPTP opening. Indeed, it has recently been shown that DJ-1 protects the heart from oxidative stress resulting from transaortic banding.16
The overexpression of DJ-1 in HL-1 cardiac cells resulted in an increase in the proportion of cells displaying predominantly elongated mitochondria, a phenotype which we have previously reported to prevent IRI-induced mitochondrial fission and to be associated with cell survival.11 Interestingly, in the DJ-1 KO murine hearts we observed increased mitochondrial fragmentation. These findings suggest that mitochondrial DJ-1 can modify mitochondrial morphology, although the mechanism through which this is achieved is unclear. The protective effect of DJ-1 against mitochondrial fragmentation has also been reported in cultured SH-SY5Y cells.17 Despite the changes in mitochondrial morphology in DJ-1 KO hearts, we did not find any difference in mitochondrial respiration rates or myocardial ATP levels, suggesting that DJ-1 is not crucial for mitochondrial function at basal conditions. Whether we would have observed mitochondrial dysfunction under conditions of IRI remains to be determined. Interestingly, experimental studies of DJ-1 in the brain have also demonstrated that the DJ-1 ablation did not affect mitochondrial respiration in mouse embryonic fibroblast cells or primary isolated mitochondria from the cerebral cortex.18 It must be appreciated that DJ-1 may protect against cell death through other mechanisms, including (1) an anti-apoptotic pathway in which it sequesters death-domain-associated protein (Daxx) to disrupt Daxx-ASK1 (apoptosis signal-regulating kinase 1) regulated apoptosis19 or (2) mitophagy.6
In summary, we have found that in the adult murine heart DJ-1 may be a novel target for protecting the myocardium against cell death from acute IRI. Experimental studies have shown that the upregulation of DJ-1 by sodium 4-phenylbutyrate protected against acute IRI in the brain.20, 21 Whether this therapeutic approach is effective against acute myocardial IRI remains to be investigated.
Materials and Methods
All animal experiments were conducted in accordance with the Animals (Scientific Procedures) Act 1986 published by the UK Home Office and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health 1996. All laboratory reagents were purchased from Sigma (Poole, UK) unless otherwise stated.
HL-1 cell plasmid transfection
All in vitro experiments were conducted using the HL-1 cardiac cell line, cultured as described in published methods.22 HL-1 cells were transfected using Fugene6 (Roche, Basel, Switzerland) according to the manufacturer’s instructions. Plasmids were: empty plasmid vector (RcCMV), mitochondrial red fluorescent protein (MtRFP), mitofusin 1 (Mfn1; Pcb6-MYC-Mfn1) (Professor L Scorrano, University of Padova, Padova, Italy), WT DJ-1 (WT DJ-1) (Dr. Z Yao and Dr. G Szabadkai, University College London, London, UK), WT DJ-1 with FLAG-tag sequence (pRK5 Flag DJ-1 WT) (Dr. Z Yao and Dr. G Szabadkai, University College London, London, UK), MitoDJ-1 (Dr. Z Yao and Dr. G Szabadkai, University College London, London, UK), L166P mutant DJ-1 (Dr. Z Yao and Dr. G Szabadkai, University College London, London, UK), Cys106A mutant DJ-1 (Professor P Kahle, University Clinics Tübingen, Tübingen, Germany), and plasmid-enhanced green fluorescent protein (Clontech, Mountain View, CA, USA). All cell experiments were conducted 24 h post transfection.
Cell death following simulated IRI in vitro
HL-1 cells were subjected to simulated IRI comprising simulated ischemia for 12 h in ischemic buffer in an air-tight hypoxic chamber and simulated reperfusion for 1 h in normoxic buffer on removal from the hypoxic chamber. Cell death was assessed in 50 cells by propidium iodide staining, and PeGFP expression was observed using fluorescence microscopy (Nikon Eclipse TE200, Nikon, Kingston Upon Thames, UK). Control cell death was determined in un-transfected HL-1 cells incubated in oxygenated normoxic buffer for 13 h. Insulin (Invitrogen, Paisley, UK) treatment (275 U/ml) administered at the onset of reperfusion was used as a positive control.
MPTP opening in vitro
MPTP opening in HL-1 cells was induced using laser-induced mitochondrial oxidative stress as previously published.11, 23 HL-1 cells were loaded with 3 μM TMRM for 15 min at 37 °C and imaged by time-scan confocal microscopy (Leica, Wetzlar, Germany). Time to MPTP opening was measured as the time taken to reach half maximum cytosolic TMRM fluorescence intensity of 15 cells for a minimum of five experiments. Sanglifehrin A (1 μM), a known MPTP inhibitor, was used as a positive control.
Mitochondrial morphology in vitro using confocal microscopy
HL-1 cell mitochondrial morphology was assessed using confocal microscopy (Leica) and MtRFP, as previously published.11 Ten randomly selected cells for a minimum of five independent experiments were imaged using a × 63 1/35 numerical aperture oil objective. Mitochondrial morphology was assessed by three blinded analyzers and defined as predominantly (>50%) elongated or fragmented. Mfn1 was used as a positive control.
DJ-1 whole-body KO mice
Mice with whole-body genetic ablation of the DJ-1 gene (B6.Cg-Park7tm1Shn/J) were originally created by Goldberg et al,24 backcrossed onto C57BL/6 background for at least 12 generations. DJ-1 KO mice were always compared against true WT littermate controls (DJ-1 WT).
Echocardiographic phenotyping
Cardiac phenotyping of DJ-1 WT and DJ-1 KO animals was performed by transthoracic two-dimensional echocardiography with Vivid 7 Dimension and 14 MHz probe (GE Healthcare, Hatfield, UK) as previously published25 to determine LV dimensions, heart rate, cardiac output, stroke volume and fractional shortening. Phenotyping was performed at baseline and 4 min following isoproterenol challenge (4 ng/g (Sigma) intraperitoneal bolus).
Myocardial ischemia–reperfusion in vivo
DJ-1 WT and DJ-1 KO animals underwent in vivo myocardial IRI comprising open-chest surgery for occlusion of left anterior descending coronary artery followed by reperfusion. Non-recovery: Anesthesia was provided by ketamine 10 mg/ml (Vetalar, Boehringer Ingelheim, Bracknell, UK), xylazine 2 mg/ml (Rompun, Bayer, Newbury, UK) and atropine 0.06 mg/ml (Sigma) at 0.1 ml/10 g and 0.1 ml doses thereafter as required. Recovery: Anesthesia was by inhaled isoflurane (1.5–2.0% vaporized in 1.5 l/min oxygen). Analgesia was provided by buprenorphine (Vetergesic, Alstoe Animal Health, York, UK) 0.1 mg/kg intramuscular at 0, 6 and 24 h post surgery. Standard recovery IRI experiments comprised 15-min stabilization, 45-min ischemia and 24-h reperfusion. IPC protocol was 5-min stabilization, 5-min ischemia and 5-min reperfusion before the index ischemic event.
MI size following in vivo IRI
At 24 h reperfusion, mice were anesthetized using non-recovery anesthetics as above, and hearts were rapidly extracted for staining with triphenyl-tetrazolium chloride and Evan’s blue for determining MI size and AAR, respectively. Quantifications were performed on transverse heart slices using ImageJ planimetry (NIH Image, Bethesda, MD, USA), and MI size was expressed as percentage of AAR (%IS/AAR).
Calcium-induced MPTP assay
Mitochondrial swelling was examined in mitochondria isolated from DJ-1 WT and DJ-1 KO hearts. Mitochondria were isolated by Trypsin (5 mg/ml) according to a modified protocol.26 Mitochondria were purified by differential centrifugation, and calcium-triggered mitochondrial-swelling assays were performed as described by Hafner et al.27 Briefly, calcium chloride (600 or 800 μM) was added to mitochondria, and optical density was measured by spectrophotometry (FLUOstar Omega, BMG Labtech, Cary, NC, USA) for 17 min. Treatment with the known MPTP inhibitor, CsA (1.0 μM), was used as a positive control.
Mitochondrial morphology by electron microscopy (EM)
DJ-1 WT and DJ-1 KO hearts at baseline or after 20-min ischemia (n≥3/group) were prepared for EM as previously published and imaged using a Jeol 1010 electron microscope (Jeol Ltd, Welwyn Garden City, UK).11 Ten images were taken for each heart. Mitochondrial elongation was assessed by comparison of interfibrillar mitochondria (IFM) to sarcomere length. Analysis was performed by two blinded analyzers on a total of 500–600 IFM.
Myocardial ATP assay
The left ventricles from DJ-1 WT and DJ-1 KO hearts were homogenized in PBS containing protease and phosphatase inhibitors and EDTA (pH 8.0) (Thermo Scientific, Epsom, UK) 1 ml/0.1 g of tissue. Myocardial ATP levels were measured using ATP bioluminescence assay kit (Sigma) in accordance with the manufacturer’s instructions where luminescence was read every 5 s for 2 min (FLUOstar Omega, BMG Labtech).
Mitochondrial respiration assay
Mitochondria were isolated from DJ-1 WT and DJ-1 KO hearts as described above. Oxygen consumption rates were measured using a high-resolution respirometer equipped with a Clark oxygen sensor (Oroboros Oxygraph, Paar KG, Graz, Austria).
Statistics
All values are expressed as mean±S.E.M. Data were analyzed by one-way analysis of variance (ANOVA) and Tukey’s multiple-comparison post-hoc test or unpaired t-test where indicated. Differences were considered significant when *P<0.05.
Abbreviations
- ATP:
-
adenosine triphosphate
- CsA:
-
ciclosporin A
- Daxx:
-
death-domain-associated protein
- EM:
-
electron microscopy
- IHD:
-
ischemic heart disease
- IPC:
-
ischemic preconditioning
- IRI:
-
ischemia–reperfusion injury
- IS/AAR:
-
infarct size/area-at-risk
- KO:
-
knockout
- LV:
-
left ventricular
- Mfn1:
-
mitofusin 1
- MitoDJ-1:
-
mitochondrial targeted DJ-1
- MI:
-
myocardial infarct
- MPTP:
-
mitochondrial permeability transition pore
- MtRFP:
-
mitochondrial red fluorescent protein
- NS:
-
not statistically significant
- TMRM:
-
tetramethyl rhodamine methyl ester
- WT:
-
wild type
References
Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 2003; 299: 256–259.
Zhang L, Shimoji M, Thomas B, Moore DJ, Yu SW, Marupudi NI et al. Mitochondrial localization of the Parkinson's disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet 2005; 14: 2063–2073.
Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H . DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep 2004; 5: 213–218.
Kinumi T, Kimata J, Taira T, Ariga H, Niki E . Cysteine-106 of DJ-1 is the most sensitive cysteine residue to hydrogen peroxide-mediated oxidation in vivo in human umbilical vein endothelial cells. Biochem Biophys Res Commun 2004; 317: 722–728.
Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S et al. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA 2004; 101: 9103–9108.
Krebiehl G, Ruckerbauer S, Burbulla LF, Kieper N, Maurer B, Waak J et al. Reduced basal autophagy and impaired mitochondrial dynamics due to loss of Parkinson's disease-associated protein DJ-1. PLoS One 2010; 5: e9367.
Aleyasin H, Rousseaux MW, Phillips M, Kim RH, Bland RJ, Callaghan S et al. The Parkinson's disease gene DJ-1 is also a key regulator of stroke-induced damage. Proc Natl Acad Sci USA 2007; 104: 18748–18753.
Herrera FE, Zucchelli S, Jezierska A, Lavina ZS, Gustincich S, Carloni P . On the oligomeric state of DJ-1 protein and its mutants associated with Parkinson disease. A combined computational and in vitro study. J. Biol. Chem 2007; 282: 24905–24914.
Macedo MG, Anar B, Bronner IF, Cannella M, Squitieri F, Bonifati V et al. The DJ-1L166P mutant protein associated with early onset Parkinson's disease is unstable and forms higher-order protein complexes. Hum Mol Genet 2003; 12: 2807–2816.
Shanmuganathan S, Hausenloy DJ, Duchen MR, Yellon DM . Mitochondrial permeability transition pore as a target for cardioprotection in the human heart. Am J Physiol Heart Circ Physiol 2005; 289: H237–H242.
Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ . Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 2010; 121: 2012–2022.
Yu HH, Xu Q, Chen HP, Wang S, Huang XS, Huang QR et al. Stable overexpression of DJ-1 protects H9c2 cells against oxidative stress under a hypoxia condition. Cell Biochem Funct 2013; 31: 643–651.
Lu HS, Chen HP, Wang S, Yu HH, Huang XS, Huang QR et al. Hypoxic preconditioning up-regulates DJ-1 protein expression in rat heart-derived H9c2 cells through the activation of extracellular-regulated kinase 1/2 pathway. Mol Cell Biochem 2012; 370: 231–240.
Aleyasin H, Rousseaux MW, Marcogliese PC, Hewitt SJ, Irrcher I, Joselin AP et al. DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc Natl Acad Sci USA 2010; 107: 3186–3191.
Kim RH, Peters M, Jang Y, Shi W, Pintilie M, Fletcher GC et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell 2005; 7: 263–273.
Billia F, Hauck L, Grothe D, Konecny F, Rao V, Kim RH et al. Parkinson-susceptibility gene DJ-1/PARK7 protects the murine heart from oxidative damage in vivo. Proc Natl Acad Sci USA 2013; 110: 6085–6090.
Kamp F, Exner N, Lutz AK, Wender N, Hegermann J, Brunner B et al. Inhibition of mitochondrial fusion by alpha-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J 2010; 29: 3571–3589.
Giaime E, Yamaguchi H, Gautier CA, Kitada T, Shen J . Loss of DJ-1 does not affect mitochondrial respiration but increases ROS production and mitochondrial permeability transition pore opening. PLoS One 2012; 7: e40501.
Junn E, Taniguchi H, Jeong BS, Zhao X, Ichijo H, Mouradian MM . Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc Natl Acad Sci USA 2005; 102: 9691–9696.
Zhou W, Bercury K, Cummiskey J, Luong N, Lebin J, Freed CR . Phenylbutyrate up-regulates the DJ-1 protein and protects neurons in cell culture and in animal models of Parkinson disease. J Biol Chem 2011; 286: 14941–14951.
Zhou W, Bercury K, Cummiskey J, Luong N, Lebin J, Freed CR . Phenylbutyrate upregulates DJ-1 and protects neurons in cell culture and in animal models of Parkinson's disease. J Biol Chem 2011; 286: 14941–14951.
Claycomb WC, Lanson Jr NA, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A et al. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 1998; 95: 2979–2984.
Davidson SM, Hausenloy D, Duchen MR, Yellon DM . Signalling via the reperfusion injury signalling kinase (RISK) pathway links closure of the mitochondrial permeability transition pore to cardioprotection. Int J Biochem Cell Biol 2006; 38: 414–419.
Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C et al. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron 2005; 45: 489–496.
Lim SY, Hausenloy DJ, Arjun S, Price AN, Davidson SM, Lythgoe MF et al. Mitochondrial cyclophilin-D as a potential therapeutic target for post-myocardial infarction heart failure. J Cell Mol Med 2011; 15: 2443–2451.
Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W et al. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res 2008; 80: 30–39.
Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A et al. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany. NY) 2010; 2: 914–923.
Acknowledgements
We thank Dr. F Giorgini and Dr. M Repici (University of Leicester, UK) for kindly providing us with the DJ-1 transgenic mice; Nicholas Davies and Abdul Mokit (Biological Services Unit, UCL) for their invaluable assistance with the DJ-1 colony; Mark Turmaine (Electron Microscopy Unit, UCL) for his help with preparing the electron microscopy sections; Dr. P Kahle (University Clinics Tübingen, Germany) for kindly providing the Cys106A DJ-1 mutant plasmid; and Professor L Scorrano (University of Padova, Italy) for the RcCMV, mtRFP and Mitofusin 1 plasmids. This work was supported by the British Heart Foundation program grant number RG/03/007 and supported by researchers at the National Institute for Health Research University College London Hospitals Biomedical Research Centre. DH is supported by British Heart Foundation Senior Clinical Research Fellowship FS/10/039/28270.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by RA Knight
Supplementary Information accompanies this paper on Cell Death and Disease website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by/3.0/
About this article
Cite this article
Dongworth, R., Mukherjee, U., Hall, A. et al. DJ-1 protects against cell death following acute cardiac ischemia–reperfusion injury. Cell Death Dis 5, e1082 (2014). https://doi.org/10.1038/cddis.2014.41
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2014.41
Keywords
This article is cited by
-
DJ-1-mediated p62 degradation delays intervertebral disc degeneration by inhibiting apoptosis of nucleus pulposus cells
Apoptosis (2023)
-
DJ-1 interacts with the ectopic ATP-synthase in endothelial cells during acute ischemia and reperfusion
Scientific Reports (2022)
-
Fasting increases susceptibility to acute myocardial ischaemia/reperfusion injury through a sirtuin-3 mediated increase in fatty acid oxidation
Scientific Reports (2022)
-
DJ-1 attenuates the glycation of mitochondrial complex I and complex III in the post-ischemic heart
Scientific Reports (2021)
-
Approaches to the Early Diagnosis of Parkinson’s Disease
Neuroscience and Behavioral Physiology (2020)