Abstract
Aim:
Oxymatrine (OMT) is the major quinolizidine alkaloid extracted from the root of Sophora flavescens Ait (the Chinese herb Kushen) and exhibits diverse pharmacological actions. In this work we investigated the effects of OMT on diabetes-associated cognitive decline (DACD) in a rat model of diabetes and explored the mechanisms of action.
Methods:
Male Wistar rats were injected with streptozotocin (65 mg/kg, ip) once to induce diabetes. The rats were then treated with vehicle or OMT (60 or 120 mg/kg per day, ip) for 7 weeks. Memory function was assessed using Morris water maze test. The levels of malondialdehyde (MDA), superoxide dismutase (SOD), glutathione (GSH), NF-κB p65 unit, TNF-α, IL-1β and caspase-3 in the cerebral cortex and hippocampus were quantified.
Results:
The diabetic rats exhibited markedly reduced body weight and increased plasma glucose level. The memory function of the rats assessed using Morris water maze test showed significant reduction in the percentage of time spent in the target quadrant and the number of times crossing the platform, coupled with markedly prolongation of escape latency and mean path length. Moreover, the rats showed oxidative stress (significantly increased MDA, decreased SOD and reduced GSH levels), as well as significant increases of NF-κB p65 unit, TNF-α, IL-1β and caspase-3 levels in the cerebral cortex and hippocampus. Chronic treatment with OMT dose-dependently reversed these behavioral, biochemical and molecular changes in the diabetic rats. However, the swimming speed had no significant difference among the control, diabetic and OMT-treated diabetic rats.
Conclusion:
Chronic treatment with OMT alleviates diabetes-associated cognitive decline in rats, which is associated with oxidative stress, inflammation and apoptotic cascades.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a serious chronic metabolic disorder and can adversely affect multiple organs owing to its long-term complications. It is estimated that 171 million people suffered from DM worldwide in 2007; this figure could be more than double by 20301. Emerging evidence suggests that DM might also have negative effects on the central nervous system (CNS), with cognitive impairment as the most common symptom2, aside from the common complications of the peripheral nervous system in diabetic patients3,4. A new term, “diabetes-associated cognitive decline (DACD)”, was proposed in 2006 to facilitate research in this field and to strengthen recognition of this disorder5. Thus, diabetes-induced cognitive impairment is a problem that is gaining increased acceptance and attention. It is essential to determine the pathophysiological changes in the generation and progression of DACD to develop potential targets for the prevention of these cognitive symptoms.
Cognitive dysfunction in diabetics appears to be caused by various factors6. Some investigations have revealed that diabetes-induced cognitive decline strongly correlates with cardiovascular disease, such as hypertension and cerebral vascular complications7,8. One report showed that diabetes-related cognitive impairment was caused by disrupted insulin signaling and glucose homeostasis in the CNS9. In another study hyperglycemia-associated microvascular changes in the brain triggered cognitive deficits in patients with type 1 diabetes (T1DM), and intensive insulin therapy in T1DM, leading to a durable improvement of glycemic control, reduced the risk of microvascular complications10. Hyperglycemia is a critical factor for the development of cognitive decline in patients with T1DM, suggesting that drugs for the alleviation of DACD could be based on their improvement of glycemic control.
Oxidative stress is involved in the pathogenesis of diabetes. Increased oxidative stress produces serious oxidative damage in the brain under diabetic conditions11. A previous investigation determined that the excessive production of oxygen free radicals and/or antioxidant deficiency in various brain regions was associated with morphological abnormalities and memory deficits12. Endothelial oxidative stress has been shown to cause serious vascular damage13. Thus, treatment with antioxidants could protect neurons against neurodegenerative conditions. In addition, increased release of inflammatory cytokines and excessive inflammation are observed in diabetics14. Activation of the nuclear transcription factor κB (NF-κB) signaling pathway was shown to induce cognitive deficits15 as well as neuronal apoptosis16 in diabetics.
Oxymatrine (OMT) is the major quinolizidine alkaloid from the root of Sophora flavescensAit (Kushen) and exhibits diverse pharmacological actions. In both preclinical and clinical investigations, it has been confirmed to possess anti-inflammatory, anti-tumor, anti-viral, anti-oxidant and anti-apoptotic properties17. A previous report demonstrated that OMT could exert a protective effect on ischemia/reperfusion damage in liver18. In colitis induced by dextran sulfate sodium, OMT was found to ameliorate the inflammatory response via reducing the expression of NF-κB in colonic mucosa19. More importantly, oral administration of OMT could significantly reduce body weight gain and blood glucose level in high fructose-fed mice, a model of fatty liver20. Therefore, it was speculated that OMT might have a protective effect on diabetes. Because DACD is the most common complication of diabetes and is closely related to inflammation, oxidative stress and apoptosis, we hypothesized that the neuroprotective effect of OMT may ameliorate the symptoms of DACD. To test this hypothesis, our experiments were designed to assess the protective effect of OMT on DACD in a rat model of diabetes.
Materials and methods
Animals
Male Wistar rats, weighing 230–250 g (obtained from the Animal Center of the Chinese Academy of Sciences, Shanghai, China), were maintained in a controlled environment (12 h:12 h day/night cycle, 50%–70% humidity, 24 °C) with free access to water and rodent chow. Great efforts were made to minimize the suffering of the animals. All experimental procedures conformed to the guidelines established by the Ministry of Health and were approved by the Animal Care Committee of Xuanwu Hospital of Capital Medical University.
Drugs and chemicals
OMT (with a purity >98%) and streptozotocin (STZ) were obtained from Sigma (St Louis, MO, USA). A glucose oxidase peroxidase diagnostic enzyme kit was purchased from India (Span Diagnostic Chemicals, India). TNF-α and IL-1β ELISA kits were supplied by R&D Systems (USA). The NF-κB p65 unit ELISA kit was obtained from Imgenex (USA). All other reagents were of analytical grade.
Induction and assessment of diabetes
Diabetes was induced in the rats by intraperitoneal injection of a single dose of 65 mg/kg STZ that was freshly dissolved in citrate buffer (pH 4.4, 0.1 mol/L). Age-matched normal rats were treated with citrate buffer only. At 48 h after the STZ injection, blood samples were collected and plasma glucose levels were measured using an enzymatic glucose oxidase peroxidase diagnostic kit. Rats with fasting plasma glucose levels higher than 250 mg/dL21 were defined as diabetic and selected for further investigation. Animals in each experiment were randomly assigned to four groups: (1) the control group (Con) (n=10), including normal rats that received saline intraperitoneally (physiological saline 0.1 mL/100 g); (2) the vehicle group (DM) (n=10), including diabetic rats that received saline intraperitoneally (physiological saline 0.1 mL/100 g); and (3–4) the OMT groups [DM+OMT(60) and DM+OMT(120)] (n=10), including diabetic rats treated with OMT at doses of 60 and 120 mg/kg, respectively. The OMT dosage and dosing frequency were selected according to previous reports22,23. OMT was freshly prepared by dissolving in saline and injected intraperitoneally once a day. The chemical structure of OMT is shown in Figure 1. Beginning on the third day of the experiment, the control and diabetic control groups were treated with the OMT vehicle through the seventh week.
The chemical structure of OMT.
After seven weeks, learning and memory functions were evaluated for 5 consecutive days in a Morris water maze. Under anesthesia induced by an intraperitoneal injection of chloral hydrate (300 mg/kg), the rats for each group were euthanized, blood samples were collected and the brains were rapidly removed. The samples were stored at −80 °C until use for biochemical measurements.
Morris water maze test
The Morris water maze tests were used after seven weeks of STZ injection. The apparatus consisted of a circular water tank (90-cm inner diameter and 50-cm height) equipped with a digital pick-up camera 180 cm above the water surface to monitor the animal behavior, a platform (6 cm in diameter) and a computer program (WaterMaze) for data analysis. The tank was partially filled with tap water and the water temperature was maintained at approximately 25±2 °C. Black non-toxic paint was used to render the water opaque. The rats were habituated to the water by being allowed to swim freely without a platform present prior to performing the water maze test. The pool was placed in the center of a large room containing various visual cues and divided into four equal quadrants, N (north), S (south), E (east), and W (west). The cues remained constant throughout the study. A translucent acrylic platform was submerged approximately 1 cm below the water surface (for the navigation test) or removed from the tank (for the spatial probe test). The water maze task was used for 5 consecutive days.
Learning test
The animals were tested with a spatial version of the Morris water maze according to a previously described method24. In brief, the rats were subjected to 4 consecutive daily training trials for 4 d, with each trial having a time limit of 60 s and with an inter-trial interval of 30 min. For each trial, the rat swam until it climbed onto the submerged platform. After climbing onto the platform, the animals were allowed to remain on the platform for 20 s. If the rat failed to find the platform within a maximum of 60 s, it was gently placed on the platform and remained there for an equivalent amount of time. The escape latency (s) and path length (cm) to find the platform were measured in each trial and averaged over three trials for each rat. Swimming speed was analyzed by dividing the path length by the time required to find the platform.
Memory test
After the final escape training, each rat was administered a spatial probe test on the 5th d to assess the extent of memory24. The platform was removed from the tank and each animal was placed in the pool from the start location at the quadrant opposite the former platform quadrant. The number of times the rat crossed the former location of the platform and the time spent in the former platform quadrant were measured at an interval of 60 s.
Biochemical assessment
The concentration of malondialdehyde (MDA), an index of lipid peroxidation, was measured in the form of thiobarbituric acid-reactive substances at 532 nm according to a previously reported method25. MDA was determined using a commercial kit (A003-1, Jiancheng Biotech Co, Nanjing, China). Cytosolic superoxide dismutase (SOD) activity was determined by the method of Kono et al26. SOD was measured using a commercial kit (A001-1, Jiancheng Biotech Co). Reduced glutathione was assayed by the method of Jollow et al27. Glutathione (GSH) levels were determined with a commercial kit (A006, Jiancheng Biotech Co). Protein content was measured using Coomassie brilliant blue28.
Measurement of NF-κB p65 unit, TNF-α, and IL-1β levels in rat brain
The p65 subunit may be positively correlated with the activation of the NF-κB pathway. The NF-κB p65 unit was measured using a commercial kit (Imgenex, USA) with colorimetric detection at 405 nm. TNF-α and IL-1β were quantified using commercial immunoassay kits (R&D Systems, USA) according to the manufacturer's instructions.
Measurement of caspase-3 activity in rat brain
The activity of caspase-3, an executioner molecule in the apoptotic cascade, was measured by cleavage of the chromogenic caspase substrate Ac-DEVD-pNA. The amount of caspase-3 was measured spectrophotometrically at 405 nm according to the manufacturer's instructions (R&D Systems, USA).
Statistical analysis
The data are expressed as mean±SD. Statistical analysis was carried out using a one-way ANOVA followed by Dunnett's test, with P<0.05 as the significant level.
Results
Effect of OMT on body weight and blood glucose levels
There was a marked reduction in the body weights of STZ-treated rats in comparison to the age-matched control group (P<0.01), as shown in Table 1. Of note, the diabetic rats exhibited a significantly elevated (589.10±3.98 mg/dL) plasma glucose level compared to the control group (112.20±1.62 mg/dL). However, after the 7-week treatment with OMT at dose of 60 or 120 mg/kg, the blood glucose levels and body weights were both reversed in diabetic rats (P<0.01).
Effect of OMT on diabetes-induced cognitive deficits
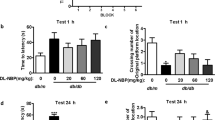
Cognitive function was assessed in the Morris water maze test (7th week). The mean escape latency for the trained animals was reduced from 60 to 20 s over the course of the 20 learning trials. No significant difference was observed between any of the groups on the first day of testing in the Morris water maze. However, beginning with the 2nd d, the transfer latency was clearly different between diabetic (53.10±1.52 s) and control (34.60±1.43 s) rats (P<0.01). Treatment with OMT (60 or 120 mg/kg) significantly diminished the mean escape latency (P<0.01), as illustrated in Figure 2A. Diabetic rats were less able to find the platform and learn its location in the four-day training session. The poorer performance was reversed by treatment with OMT at dose of 60 or 120 mg/kg, as evidenced by the decrease in latency from the 2nd d of training (P<0.01). The results, shown in Figure 2B, also indicated a significant increase in mean path length in the diabetic group for four consecutive days of training compared with the controls (P<0.01). However, treatment with OMT at dose of 60 or 120 mg/kg was associated with a marked reduction in this value compared to the vehicle-treated diabetic rats (P<0.01).
Effects of OMT on (A) spatial memory acquisition phase, (B) mean path length, (C) mean percentage of time spent in the target quadrant, (D) the number of times of crossing platform and (E) swimming speed in control and diabetic rats (n=10, mean±SD). cP<0.01 compared with Con group. fP<0.01 compared with DM group. Con, control; DM, diabetes; DM+OMT (60), oxymatrine (60 mg/kg)-treated; DM+OMT (120), oxymatrine (120 mg/kg)-treated.
The probe trial of the Morris water maze test was conducted to investigate how well the animals had learned and consolidated the platform location. Animals exhibited a significant difference (P<0.01) during the 4 d of training. There was a decline in the time spent in the target quadrant in diabetic rats compared with the control group (P<0.01), as shown in Figure 2C. Treatment with OMT (60 or 120 mg/kg) was associated with dramatically more time (P<0.01) spent in the target quadrant compared to the diabetic group. Similarly, as indicated in Figure 2D, the number of times the animals crossed the former platform location was also lower in diabetic rats (P<0.01) compared to the control group. Nevertheless, this index was significantly improved in diabetic rats after treatment with OMT (60 or 120 mg/kg) (P<0.01). As shown in Figure 2E, there was no significant difference in swimming speed among the groups across the four training days.
Effect of OMT on diabetes-induced changes in oxidative stress
Table 2 shows that STZ-induced diabetes significantly increased MDA levels in the cerebral cortex (2.05-fold) and the hippocampus (1.78-fold) compared to the age-matched control (P<0.01). However, chronic administration of OMT (60 and 120 mg/kg) prevented the increase in MDA content in the brains of diabetic rats (P<0.01). In addition, SOD activity and glutathione levels were lower in the cerebral cortex and hippocampus of diabetic animals (P<0.01) compared with the control group after 7 weeks. This reduction was significantly and dose-dependently reversed by OMT treatment in different areas in the brain of STZ-treated rats (P<0.01).
Effect of OMT on diabetes-induced changes in NF-κB p65 subunit, TNF-α and IL-1β levels
As shown in Figure 3A, the NF-κB p65 subunit was significantly elevated in the cerebral cortex (3.61-fold) and the hippocampus (3.69-fold) of diabetic rats after 7 weeks. Nevertheless, chronic treatment with OMT (60 or 120 mg/kg) significantly suppressed the elevation in different brain areas of STZ-injected rats (P<0.01). Likewise, levels of TNF-α (4.95-fold in the cerebral cortex and 5.75-fold in the hippocampus) and IL-1β (2.93-fold in the cerebral cortex and 3.01-fold in the hippocampus) were elevated in the diabetic animals after 7 weeks (Figure 3B and 3C, P<0.01). Treatment with OMT (60 or 120 mg/kg) markedly and dose-dependently inhibited TNF-α and IL-1β levels in different areas of the brain of STZ-injected rats (P<0.01).
Effects of OMT on levels of (A) NF-κB p65 subunit, (B) TNF-α and (C) IL-1β in the cerebral cortex and hippocampus of control and diabetic rats (n=10, mean±SD). cP<0.01 compared with Con group. fP<0.01 compared with DM group. Con, control; DM, diabetes; DM+OMT (60), oxymatrine (60 mg/kg)-treated; DM+OMT (120), oxymatrine (120 mg/kg)-treated.
Effect of OMT on diabetes-induced changes in caspase-3 activity
Figure 4 shows that caspase-3 levels were significantly elevated in the cerebral cortex (8.57-fold) and the hippocampus (9.45-fold) of diabetic animals after seven weeks (P<0.01). However, treatment with OMT (60 or 120 mg/kg) produced a more pronounced attenuation of caspase-3 activity in different regions of the brain of STZ-treated rat (P<0.01).
Effects of OMT on caspase-3 activity in the cerebral cortex and hippocampus of control and diabetic rats (n=10, mean±SD). cP<0.01 compared with Con group. fP<0.01 compared with DM group. Con, control; DM, diabetes; DM+OMT (60), oxymatrine (60 mg/kg)-treated; DM+OMT (120), oxymatrine (120 mg/kg)-treated.
Discussion
In humans with diabetes, chronic hyperglycemia plays a role in the high incidence of progressive dementia29. The potential mechanisms for this phenomenon probably include direct effects of hypo- or hyperglycemia, as well as indirect effects via cerebrovascular changes30. This investigation analyzed the role of OMT on the behavioral, biochemical and molecular functions of the brain in diabetic rats. The results show that OMT can reduce blood glucose level, improve cognitive function, inhibit oxidative stress, attenuate inflammatory responses and suppress neuronal apoptosis. A previous investigation suggested that OMT could exert protection against excitotoxicity following ischemia, which partially agrees with our findings31.
High blood glucose level is believed to cause neuronal damage. Direct glucose toxicity in the neurons is induced by elevated intracellular glucose oxidation, which leads to an increase in the production of reactive species32. Endothelial oxidative stress has been shown to cause serious vascular damage13. A recent report has revealed that oxidative damage to rat synapses induces cognitive deficits33, suggesting a critical role for oxidative stress in neuronal damage. ROS may be a critical factor, exacerbating cellular damage during diabetes. Under physiological circumstances, basal amount of ROS plays a crucial role in many important physiological processes34 and can be quickly scavenged by endogenous antioxidant enzymes such as SOD and low-molecular-weight antioxidants such as GSH. However, an excessive production of ROS under diabetic conditions contributes to a reduced capability of the natural antioxidant systems, leading to neuronal apoptosis. Our investigation found that the levels of MDA, SOD, and GSH increased in the cerebral cortex and hippocampus of diabetic rats, consistent with a previous report35. In addition, chronic treatment with OMT returned the values for MDA, SOD, and GSH toward their control values. This implies that the attenuation of DACD by OMT is associated with its anti-oxidant properties.
The Morris water maze test is one of the most widely accepted behavioral tests monitoring spatial learning and memory capabilities. Our study illustrated that OMT treatment significantly prevents learning and memory deficits in diabetic rats by decreasing escape latency and mean path length and increasing the percentage of time spent in the target quadrant and the number of times crossing the platform. The swimming speed did not differ among the groups. This result implies that the effect of OMT on learning and memory functions is not due to sensorimotor abnormality. A previous investigation demonstrated that OMT could improve the learning and memory performance of D(+)-galactose-induced aging mice36, which is consistent with the results described here.
Pro-inflammatory cytokines are elevated in several neuropathological states associated with learning and memory functions. The evidence suggests that TNF-α could inhibit the generation of long-term potentiation (LTP) in the dentate gyrus subfield of the rat hippocampus. The inhibition of LTP by TNF-α is mainly dependent upon the activation of tumor necrosis factor receptor 1 (TNFR1) and mGlu5-receptors, in addition to ryanodine-sensitive intracellular calcium stores. Under chronic hyperglycemia, endogenous TNF-α production is accelerated in microvascular and neural tissues, which may consequently increase microvascular permeability, hypercoagulability and neuronal damage, finally initiating and promoting the development of diabetic microangiopathy and encephalopathy. NF-κB might be one of the crucial regulators of inflammatory damage37. NF-κB p65 is the major subunit that is sequestered in the cytoplasm and is rendered inactive through its association with inhibitory molecules, including IκB. Proinflammatory cytokine or lipopolysaccharide (LPS) stimulation induces a rapid degradation of IκB proteins, causing the release and nuclear translocation of NF-κB for gene regulation. Evidence suggests that mitochondrial ROS could modulate TNF α-mediated NF-κB activation, inducing apoptotic cell death38. Collectively, the emerging consensus is that ROS, TNF-α, and IL-1β are modulators of NF-κB signaling and caspase-3 activation in different regions of the diabetic rat brain39. Further activation of the NF-κB signaling pathway did not improve learning and memory functions in the Morris water maze task in the diabetic animals. Our results indicated that OMT significantly and dose-dependently inhibited NF-κB signaling by suppressing oxidative stress and inflammation. Consistent with our results, a recent investigation has revealed the prevention of NF-κB nuclear translocation and subsequent attenuation of inflammation in an acute model of intestinal injury in vivo40. In addition, it has been previously reported that OMT suppresses IL-1β mRNA expression in human periodontal ligament cells stimulated by LPS, which is in partial agreement with our current work41.
The TNF family is also activated by releasing mitochondrial apoptosis-inducing factors, leading to ROS generation. To better understand the pathway that causes apoptosis in diabetes, we investigated the activity of apoptosis-regulatory proteins in different areas of the brain of diabetic rats. Caspases are specifically activated by apoptotic stimuli, and caspase-3 is conceived as an executioner in apoptotic cascades. In the present study, diabetics exhibited a marked elevation of caspase-3 activity in the cerebral cortex and hippocampus. This effect was prevented by OMT treatment, suggesting that OMT reduced neuronal cell death in a rat model of diabetes. In contrast, previous investigations demonstrated that OMT induced caspase-3-dependent apoptosis in a human melanoma cell line (A375)42 and in human pancreatic cancer PANC-1 cells43. These results may reflect differences between in vitro and in vivo studies, as well as different doses of OMT.
In conclusion, OMT provides beneficial effects by lowering blood glucose, improving learning and memory functions, reducing oxidative stress, inhibiting the TNF-α-induced NF-κB signaling pathway and diminishing caspase-3 activity in diabetic rats. These findings point toward the potential of OMT as an adjuvant therapy to conventional anti-hyperglycemic and anti-DACD regimens.
Author contribution
Jian-ping JIA contributed to the study design; Suo-bin WANG performed the research and conducted the data analysis; and Jian-ping JIA wrote the manuscript.
References
McCrimmon RJ, Ryan CM, Frier BM . Diabetes and cognitive dysfunction. Lancet 2012; 379: 2291–9.
Biessels GJ, Deary IJ, Ryan CM . Cognition and diabetes: a lifespan perspective. Lancet Neurol 2008; 7: 184–90.
Bloomgarden ZT . Diabetic neuropathy. Diabetes Care 2007; 30: 1027–32.
Dobretsov M, Romanovsky D, Stimers JR . Early diabetic neuropathy: triggers and mechanisms. World J Gastroenterol 2007; 13: 175–91.
Mijnhout GS, Scheltens P, Diamant M, Biessels GJ, Wessels AM, Simsek S, et al. Diabetic encephalopathy: A concept in need of a definition. Diabetologia 2006; 49: 1447–8.
Kodl CT, Seaquist ER . Cognitive dysfunction and diabetes mellitus. Endocr Rev 2008; 29: 494–511.
Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB . Obesity, diabetes and cognitive deficit: the framingham heart study. Neurobiol Aging 2005; 26: 11–6.
Brown CM, Marthol H, Zikeli U, Ziegler D, Hilz MJ . A simple deep breathing test reveals altered cerebral autoregulation in type 2 diabetic patients. Diabetologia 2008; 51: 756–61.
Reagan LP, Grillo CA, Piroli GG . The As and Ds of stress: metabolic, morphological and behavioral consequences. Eur J Pharmacol 2008; 585: 64–75.
Wessels AM, Scheltens P, Barkhof F, Heine RJ . Hyperglycaemia as a determinant of cognitive decline in patients with type 1 diabetes. Eur J Pharmacol 2008; 585: 88–96.
Sharma B, Singh N . Behavioral and biochemical investigations to explore pharmacological potential of PPAR-gamma agonists in vascular dementia of diabetic rats. Pharmacol Biochem Behav 2011; 100: 320–9.
Fukui K, Onodera K, Shinkai T, Suzuki S, Urano S . Impairment of learning and memory in rats caused by oxidative stress and aging, and changes in antioxidative defense systems. Ann N Y Acad Sci 2001; 928: 168–75.
Angeli JK, Pereira CA, de Oliveira Faria T, Stefanon I, Padilha AS, Vassallo DV . Cadmium exposure induces vascular injury due to endothelial oxidative stress: the role of local angiotensin II and COX-2. Free Radic Biol Med 2013; 65: 838–48.
Umegaki H . Pathophysiology of cognitive dysfunction in older people with type 2 diabetes: vascular changes or neurodegeneration? Age Ageing 2010; 39: 8–10.
Kuhad A, Bishnoi M, Tiwari V, Chopra K . Suppression of NF-kappabeta signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol Biochem Behav 2009; 92: 251–9.
Mastrocola R, Restivo F, Vercellinatto I, Danni O, Brignardello E, Aragno M, et al. Oxidative and nitrosative stress in brain mitochondria of diabetic rats. J Endocrinol 2005; 187: 37–44.
Hong-Li S, Lei L, Lei S, Dan Z, De-Li D, Guo-Fen Q, et al. Cardioprotective effects and underlying mechanisms of oxymatrine against Ischemic myocardial injuries of rats. Phytother Res 2008; 22: 985–9.
Jiang H, Meng F, Li J, Sun X . Anti-apoptosis effects of oxymatrine protect the liver from warm ischemia reperfusion injury in rats. World J Surg 2005; 29: 1397–401.
Zheng P, Niu FL, Liu WZ, Shi Y, Lu LG . Anti-inflammatory mechanism of oxymatrine in dextran sulfate sodium-induced colitis of rats. World J Gastroenterol 2005; 11: 4912–5.
Zeng XY, Zhou X, Xu J, Chan SM, Xue CL, Molero JC, et al. Screening for the efficacy on lipid accumulation in 3T3-L1 cells is an effective tool for the identification of new anti-diabetic compounds. Biochem Pharmacol 2012; 84: 830–7.
Kuhad A, Chopra K . Effect of sesamol on diabetes-associated cognitive decline in rats. Exp Brain Res 2008; 185: 411–20.
Dong XQ, Yu WH, Hu YY, Zhang ZY, Huang M . Oxymatrine reduces neuronal cell apoptosis by inhibiting Toll-like receptor 4/nuclear factor kappa-B-dependent inflammatory responses in traumatic rat brain injury. Inflamm Res 2011; 60: 533–9.
Huang M, Hu YY, Dong XQ, Xu QP, Yu WH, Zhang ZY . The protective role of oxymatrine on neuronal cell apoptosis in the hemorrhagic rat brain. J Ethnopharmacol 2012; 143: 228–35.
Morris RG, Garrud P, Rawlins JN, O'Keefe J . Place navigation impaired in rats with hippocampal lesions. Nature 1982; 297: 681–3.
Wills ED . Mechanisms of lipid peroxide formation in animal tissues. Biochem J 1966; 99: 667–76.
Kono Y . Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 1978; 186: 189–95.
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR . Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974; 11: 151–69.
Cao Y, Mao X, Sun C, Zheng P, Gao J, Wang X, et al. Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Res Bull 2011; 85: 396–402.
Ryan CM, Geckle MO, Orchard TJ . Cognitive efficiency declines over time in adults with Type 1 diabetes: effects of micro- and macrovascular complications. Diabetologia 2003; 46: 940–8.
Lobnig BM, Kromeke O, Optenhostert-Porst C, Wolf OT . Hippocampal volume and cognitive performance in long-standing Type 1 diabetic patients without macrovascular complications. Diabet Med 2006; 23: 32–9.
Zhang K, Li YJ, Yang Q, Gerile O, Yang L, Li XB, et al. Neuroprotective effects of oxymatrine against excitotoxicity partially through down-regulation of NR2B-containing NMDA receptors. Phytomedicine 2013; 20: 343–50.
Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000; 404: 787–90.
Tuzcu M, Baydas G . Effect of melatonin and vitamin E on diabetes-induced learning and memory impairment in rats. Eur J Pharmacol 2006; 537: 106–10.
Droge W . Free radicals in the physiological control of cell function. Physiol Rev 2002; 82: 47–95.
Waisundara VY, Hsu A, Tan BK, Huang D . Baicalin improves antioxidant status of streptozotocin-induced diabetic Wistar rats. J Agric Food Chem 2009; 57: 4096–102.
Zi XH, Zhou W, Chen Q, Li M, Gu SL . The effect of oxymatrine on aging mice caused by D+ -galactose. Zhong Yao Cai 2012; 35: 1455–9.
Hayden MS, Ghosh S . Signaling to NF-kappaB. Genes Dev 2004; 18: 2195–224.
Hughes G, Murphy MP, Ledgerwood EC . Mitochondrial reactive oxygen species regulate the temporal activation of nuclear factor kappaB to modulate tumour necrosis factor-induced apoptosis: evidence from mitochondria-targeted antioxidants. Biochem J 2005; 389: 83–9.
Kim EK, Kwon KB, Han MJ, Song MY, Lee JH, Lv N, et al. Coptidis rhizoma extract protects against cytokine-induced death of pancreatic beta-cells through suppression of NF-kappaB activation. Exp Mol Med 2007; 39: 149–59.
Guzman JR, Koo JS, Goldsmith JR, Muhlbauer M, Narula A, Jobin C . Oxymatrine prevents NF-kappaB nuclear translocation and ameliorates acute intestinal inflammation. Sci Rep 2013; 3: 1629.
Wu Y, Chen L, Luo K, Yan FH . The effects of oxymatrine on expression of interleukin-6 and interleukin-1beta mRNA of human periodontal ligament cell stimulated by lipopolysaccharides. Hua Xi Kou Qiang Yi Xue Za Zhi 2010; 28: 656–9.
Zhang Y, Liu H, Jin J, Zhu X, Lu L, Jiang H . The role of endogenous reactive oxygen species in oxymatrine-induced caspase-3-dependent apoptosis in human melanoma A375 cells. Anticancer Drugs 2010; 21: 494–501.
Ling Q, Xu X, Wei X, Wang W, Zhou B, Wang B, et al. Oxymatrine induces human pancreatic cancer PANC-1 cells apoptosis via regulating expression of Bcl-2 and IAP families, and releasing of cytochrome c. J Exp Clin Cancer Res 2011; 30: 66.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Sb., Jia, Jp. Oxymatrine attenuates diabetes-associated cognitive deficits in rats. Acta Pharmacol Sin 35, 331–338 (2014). https://doi.org/10.1038/aps.2013.158
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2013.158
Keywords
This article is cited by
-
Ameliorative and Neuroprotective Effect of Core-Shell Type Se@Au Conjugated Hesperidin Nanoparticles in Diabetes-Induced Cognitive Impairment
Molecular Neurobiology (2023)
-
L-carnitine Modulates Cognitive Impairment Induced by Doxorubicin and Cyclophosphamide in Rats; Insights to Oxidative Stress, Inflammation, Synaptic Plasticity, Liver/brain, and Kidney/brain Axes
Journal of Neuroimmune Pharmacology (2023)
-
Oxymatrine Alleviates Hyperglycemic Cerebral Ischemia/Reperfusion Injury via Protecting Microvessel
Neurochemical Research (2022)
-
Tetramethylpyrazine Attenuates Cognitive Impairment Via Suppressing Oxidative Stress, Neuroinflammation, and Apoptosis in Type 2 Diabetic Rats
Neurochemical Research (2022)
-
Curcumin and its analog alleviate diabetes-induced damages by regulating inflammation and oxidative stress in brain of diabetic rats
Diabetology & Metabolic Syndrome (2021)