Abstract
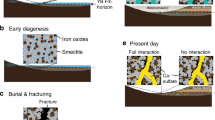
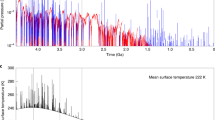
Observations from in situ experiments and planetary orbiters have shown that the sedimentary rocks found at Meridiani Planum, Mars were formed in the presence of acidic surface waters1,2,3,4. The water was thought to be brought to the surface by groundwater upwelling5,6, and may represent the last vestiges of the widespread occurrence of liquid water on Mars. However, it is unclear why the surface waters were acidic. Here we use geochemical calculations, constrained by chemical and mineralogical data from the Mars Exploration Rover Opportunity7,8,9,10, to show that Fe oxidation and the precipitation of oxidized iron (Fe3+) minerals generate excess acid with respect to the amount of base anions available in the rocks present in outcrop. We suggest that subsurface waters of near-neutral pH and rich in Fe2+ were rapidly acidified as iron was oxidized on exposure to O2 or photo-oxidized by ultraviolet radiation at the martian surface. Temporal variation in surface acidity would have been controlled by the availability of liquid water, and as such, low-pH fluids could be a natural consequence of the aridification of the martian surface. Finally, because iron oxidation at Meridiani would have generated large amounts of gaseous H2, ultimately derived from the reduction of H2O, we conclude that surface geochemical processes would have affected the redox state of the early martian atmosphere.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Grotzinger, J. P. et al. Stratigraphy and sedimentology of a dry to wet eolian depositional system, Burns formation, Meridiani Planum, Mars. Earth Planet. Sci. Lett. 240, 11–72 (2005).
Squyres, S. W. et al. Exploration of Victoria Crater by the Mars rover Opportunity. Science 324, 1058–1061 (2009).
McLennan, S. M. et al. Provenance and diagenesis of the evaporite-bearing Burns formation, Meridiani Planum, Mars. Earth Planet. Sci. Lett. 240, 95–121 (2005).
Tosca, N. J. et al. Geochemical modelling of evaporation processes on Mars: Insight from the sedimentary record at Meridiani Planum. Earth Planet. Sci. Lett. 240, 122–148 (2005).
Andrews-Hanna, J. C., Phillips, R. J. & Zuber, M. T. Meridiani Planum and the global hydrology of Mars. Nature 446, 163–166 (2007).
Andrews-Hanna, J. C., Zuber, M. T., Arvidson, R. E. & Wiseman, S. J. Early Mars Hydrology: 1. The Meridiani playa deposits and the sedimentary record of Arabia Terra. J. Geophys. Res. 10.1029/2009JE003485 (in the press).
Gellert, R. & Rieder, R. MER APXS Oxide Abundance Archive, NASA Planetary Data System, MER1/MER2-M-APXS-5-OXIDE-SCI-V1.0 (MER APXS Oxide Abundance Archive, NASA Planetary Data System, MER1/MER2-M-APXS-5-OXIDE-SCI-V1.0, 2006).
Rieder, R. et al. Chemistry of rocks and soils at Meridiani Planum from the alpha particle X-ray spectrometer. Science 306, 1746–1749 (2004).
Klingelhofer, G. et al. Jarosite and hematite at Meridiani Planum from Opportunity’s Mossbauer spectrometer. Science 306, 1740–1745 (2004).
Morris, R. V. et al. Mossbauer mineralogy of rock, soil, and dust at Meridiani Planum, Mars: Opportunity’s journey across sulphate-rich outcrop, basaltic sand and dust, and hematite lag deposits. J. Geophys. Res. 111, E12S15 (2006).
Burns, R. G. & Fisher, D. S. Iron–sulfur mineralogy of Mars–magmatic evolution and chemical-weathering products. J. Geophys. Res. 95, 14415–14421 (1990).
Bigham, J. M. & Nordstrom, D. K. in Iron and Aluminum Hydroxysulphates from Acid Sulphate Waters in Sulphate Minerals Vol. 40 (eds Alpers, C. N., Jambor, J. L. & Nordstrom, D. K.) 351–403 (Mineralogical Society of America, 2000).
Tosca, N. J. & McLennan, S. M. Experimental constraints on the evaporation of partially oxidized acid-sulphate waters at the martian surface. Geochim. Cosmochim. Acta 73, 1205–1222 (2009).
Burns, R. G. Rates and mechanisms of chemical weathering of ferromagnesian silicate minerals on Mars. Geochim. Cosmochim. Acta 57, 4555–4574 (1993).
Burns, R. G. & Fisher, D. S. Rates of oxidative weathering on the surface of Mars. J. Geophys. Res. 98, 3365–3372 (1993).
Halevy, I., Zuber, M. T. & Schrag, D. P. A sulfur dioxide climate feedback on early Mars. Science 318, 1903–1907 (2007).
Knauth, L. P., Burt, D. M. & Wohletz, K. H. Impact origin of sediments at the Opportunity landing site on Mars. Nature 438, 1123–1128 (2005).
McCollom, T. M. & Hynek, B. M. A volcanic environment for bedrock diagenesis at Meridiani Planum on Mars. Nature 438, 1129–1131 (2005).
Niles, P. B. & Michalski, J. Meridiani Planum sediments on Mars formed through weathering in massive ice deposits. Nature Geosci. 2, 215–220 (2009).
Jortner, J. & Stein, G. Photochemical evolution of hydrogen from aqueous solutions of ferrous ions.1. Reaction mechanism at low pH. J. Phys. Chem. 66, 1258–1264 (1962).
Singer, P. C. & Stumm, W. Acidic mine drainage: The rate determining step. Science 167, 1121–1123 (1970).
Catling, D. C., Zahnle, K. J. & McKay, C. Biogenic methane, hydrogen escape, and the irreversible oxidation of early Earth. Science 293, 839–843 (2001).
Yung, Y. L. et al. HDO in the martian atmosphere—implications for the abundance of crustal water. Icarus 76, 146–159 (1988).
Tian, F., Kasting, J. F. & Solomon, S. C. Thermal escape of carbon from the early martian atmosphere. Geophys. Res. Lett. 36, L02205 (2009).
Jakosky, B. M. & Phillips, R. J. Mars’ volatile and climate history. Nature 412, 237–244 (2001).
Jakosky, B. M. & Jones, J. H. The history of martian volatiles. Rev. Geophys. 35, 1–16 (1997).
Lammer, H. et al. Atmospheric escape and evolution of terrestrial planets and satellites. Space Sci. Rev. 139, 399–436 (2008).
Bibring, J. P. et al. Global mineralogical and aqueous mars history derived from OMEGA/Mars express data. Science 312, 400–404 (2006).
Clark, B. C. et al. Chemistry and mineralogy of outcrops at Meridiani Planum. Earth Planet. Sci. Lett. 240, 73–94 (2005).
Glotch, T. D. et al. Mineralogy of the light-toned outcrop at Meridiani Planum as seen by the Miniature Thermal Emission Spectrometer and implications for its formation. J. Geophys. Res. 111, E12S03 (2006).
Acknowledgements
Research was carried out at the Jet Propulsion Laboratory, California Institute of Technology, under a contract with the National Aeronautics and Space Administration (J.A.H., R.E.M.). This work was also supported by the California Institute of Technology (W.W.F.) and by an Origins Initiative Postdoctoral Fellowship (N.J.T.). The authors thank Y. Yung, N. Heavens and J. Wilson for constructive comments.
Author information
Authors and Affiliations
Contributions
J.A.H. carried out data analysis, modelling and wrote the paper, W.W.F. and J.A.H. conceived the study, N.J.T. and R.E.M. contributed to modelling and W.W.F., N.J.T. and R.E.M. contributed to writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 429 kb)
Rights and permissions
About this article
Cite this article
Hurowitz, J., Fischer, W., Tosca, N. et al. Origin of acidic surface waters and the evolution of atmospheric chemistry on early Mars. Nature Geosci 3, 323–326 (2010). https://doi.org/10.1038/ngeo831
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo831
This article is cited by
-
Atmospheric formaldehyde production on early Mars leading to a potential formation of bio-important molecules
Scientific Reports (2024)
-
Unveiling microbial preservation under hyperacidic and oxidizing conditions in the Oligocene Rio Tinto deposit
Scientific Reports (2021)
-
A coupled model of episodic warming, oxidation and geochemical transitions on early Mars
Nature Geoscience (2021)
-
Anoxic chemical weathering under a reducing greenhouse on early Mars
Nature Astronomy (2021)
-
Did Mars Possess a Dense Atmosphere During the First $\sim400$ Million Years?
Space Science Reviews (2021)