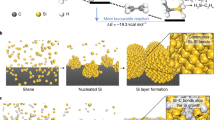
Abstract
Nanostructuring has been shown to be fruitful in addressing the problems of high-capacity Si anodes. However, issues with the high cost and poor Coulombic efficiencies of nanostructured Si still need to be resolved. Si microparticles are a low-cost alternative but, unlike Si nanoparticles, suffer from unavoidable particle fracture during electrochemical cycling, thus making stable cycling in a real battery impractical. Here we introduce a method to encapsulate Si microparticles (∼1–3 µm) using conformally synthesized cages of multilayered graphene. The graphene cage acts as a mechanically strong and flexible buffer during deep galvanostatic cycling, allowing the microparticles to expand and fracture within the cage while retaining electrical connectivity on both the particle and electrode level. Furthermore, the chemically inert graphene cage forms a stable solid electrolyte interface, minimizing irreversible consumption of lithium ions and rapidly increasing the Coulombic efficiency in the early cycles. We show that even in a full-cell electrochemical test, for which the requirements of stable cycling are stringent, stable cycling (100 cycles; 90% capacity retention) is achieved with the graphene-caged Si microparticles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Aricò, A. S., Bruce, P., Scrosati, B., Tarascon, J.-M. & van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nature Mater. 4, 366–377 (2005).
Armand, M. & Tarascon, J.-M. Building better batteries. Nature 451, 652–657 (2008).
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nature Mater. 11, 19–29 (2012).
Chan, C. K. et al. High-performance lithium battery anodes using silicon nanowires. Nature Nanotech. 3, 31–35 (2008).
Yang, S., Zavalij, P. Y. & Whittingham, M. S. Anodes for lithium batteries: tin revisited. Electrochem. Commun. 5, 587–590 (2003).
Zheng, G. et al. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nature Nanotech. 9, 618–623 (2014).
Qian, J. et al. High rate and stable cycling of lithium metal anode. Nature Commun. 6, 6362 (2015).
Ogasawara, T., Débart, A., Holzapfel, M., Novák, P. & Bruce, P. G. Rechargeable Li2O2 electrode for lithium batteries. J. Am. Chem. Soc. 128, 1390–1393 (2006).
Ji, X., Lee, K. T. & Nazar, L. F. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nature Mater. 8, 500–506 (2009).
Lu, Y.-C., Gasteiger, H. A., Crumlin, E., McGuire, R. & Shao-Horn, Y. Electrocatalytic activity studies of select metal surfaces and implications in Li–air batteries. J. Electrochem. Soc. 157, A1025 (2010).
Seh, Z. W. et al. Sulphur–TiO2 yolk–shell nanoarchitecture with internal void space for long-cycle lithium–sulphur batteries. Nature Commun. 4, 1331 (2013).
Beaulieu, L. Y., Eberman, K. W., Turner, R. L., Krause, L. J. & Dahn, J. R. Colossal reversible volume changes in lithium alloys. Electrochem. Solid-State Lett. 4, A137–A140 (2001).
Obrovac, M. N. & Christensen, L. Structural changes in silicon anodes during lithium insertion/extraction. Electrochem. Solid-State Lett. 7, A93–A96 (2004).
Obrovac, M. N., Christensen, L., Le, D. B. & Dahn, J. R. Alloy design for lithium-ion battery anodes. J. Electrochem. Soc. 154, A849–A855 (2007).
Cui, L.-F., Ruffo, R., Chan, C. K., Peng, H. & Cui, Y. Crystalline-amorphous core–shell silicon nanowires for high capacity and high current battery electrodes. Nano Lett. 9, 491–495 (2008).
Zhou, S., Liu, X. & Wang, D. Si/TiSi2 heteronanostructures as high-capacity anode material for Li ion batteries. Nano Lett. 10, 860–863 (2010).
Yao, Y. et al. Interconnected silicon hollow nanospheres for lithium-ion battery anodes with long cycle life. Nano Lett. 11, 2949–2954 (2011).
Park, M.-H. et al. Silicon nanotube battery anodes. Nano Lett. 9, 3844–3847 (2009).
Xiao, J. et al. Stabilization of silicon anode for Li-ion batteries. J. Electrochem. Soc. 157, A1047–A1051 (2010).
Yi, R., Dai, F., Gordin, M. L., Chen, S. & Wang, D. Micro-sized Si–C composite with interconnected nanoscale building blocks as high-performance anodes for practical application in lithium-ion batteries. Adv. Energy Mater. 3, 295–300 (2013).
Ge, M., Rong, J., Fang, X. & Zhou, C. Porous doped silicon nanowires for lithium ion battery anode with long cycle life. Nano Lett. 12, 2318–2323 (2012).
Lu, Z. et al. Nonfilling carbon coating of porous silicon micrometer-sized particles for high-performance lithium battery anodes. ACS Nano 9, 2540–2547 (2015).
Magasinski, A. et al. High-performance lithium-ion anodes using a hierarchical bottom-up approach. Nature Mater. 9, 353–358 (2010).
Ji, L. et al. Graphene/Si multilayer structure anodes for advanced half and full lithium-ion cells. Nano Energy 1, 164–171 (2012).
Ren, J. et al. Silicon–graphene composite anodes for high-energy lithium batteries. Energy Technol. 1, 77–84 (2013).
Son, I. H. et al. Silicon carbide-free graphene growth on silicon for lithium-ion battery with high volumetric energy density. Nature Commun. 6, 7393 (2015).
Wu, H. et al. Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control. Nature Nanotech. 7, 310–315 (2012).
Liu, N. et al. A yolk-shell design for stabilized and scalable Li-ion battery alloy anodes. Nano Lett. 12, 3315–3321 (2012).
Liu, N. et al. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nature Nanotech. 9, 187–192 (2014).
Kovalenko, I. et al. A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 334, 75–79 (2011).
Wu, H. et al. Stable Li-ion battery anodes by in-situ polymerization of conducting hydrogel to conformally coat silicon nanoparticles. Nature Commun. 4, 1943 (2013).
Wang, C. et al. Self-healing chemistry enables the stable operation of silicon microparticle anodes for high-energy lithium-ion batteries. Nature Chem. 5, 1042–1048 (2013).
Kim, H., Seo, M., Park, M. & Cho, J. A critical size of silicon nano-anodes for lithium rechargeable batteries. Angew. Chem. Int. Ed. 49, 2146–2149 (2010).
Lee, S. W., McDowell, M. T., Choi, J. W. & Cui, Y. Anomalous shape changes of silicon nanopillars by electrochemical lithiation. Nano Lett. 11, 3034–3039 (2011).
Liu, X. H. et al. Anisotropic swelling and fracture of silicon nanowires during lithiation. Nano Lett. 11, 3312–3318 (2011).
McMillan, R., Slegr, H., Shu, Z. X. & Wang, W. Fluoroethylene carbonate electrolyte and its use in lithium ion batteries with graphite anodes. J. Power Sources 81–82, 20–26 (1999).
Profatilova, I. A., Kim, S.-S. & Choi, N.-S. Enhanced thermal properties of the solid electrolyte interphase formed on graphite in an electrolyte with fluoroethylene carbonate. Electrochim. Acta 54, 4445–4450 (2009).
Jeong, S.-K. et al. Surface film formation on a graphite negative electrode in lithium-ion batteries: atomic force microscopy study on the effects of film-forming additives in propylene carbonate solutions. Langmuir 17, 8281–8286 (2001).
Schlesinger, M. & Paunovic, M. Modern Electroplating Vol. 55 (Wiley, 2011).
Nagakura, S. Study of metallic carbides by electron diffraction part I. Formation and decomposition of nickel carbide. J. Phys. Soc. Jpn 12, 482–494 (1957).
Yoon, S.-M. et al. Synthesis of multilayer graphene balls by carbon segregation from nickel nanoparticles. ACS Nano 6, 6803–6811 (2012).
Li, X., Cai, W., Colombo, L. & Ruoff, R. S. Evolution of graphene growth on Ni and Cu by carbon isotope labeling. Nano Lett. 9, 4268–4272 (2009).
Ferrari, A. C. & Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 61, 14095–14107 (2000).
Huang, J. Y. et al. In situ observation of the electrochemical lithiation of a single SnO2 nanowire electrode. Science 330, 1515–1520 (2010).
McDowell, M. T. et al. In situ TEM of two-phase lithiation of amorphous silicon nanospheres. Nano Lett. 13, 758–764 (2013).
Smith, A. J., Burns, J. C., Trussler, S. & Dahn, J. R. Precision measurements of the coulombic efficiency of lithium-ion batteries and of electrode materials for lithium-ion batteries. J. Electrochem. Soc. 157, A196–A202 (2010).
Chou, S.-L. et al. Enhanced reversible lithium storage in a nanosize silicon/graphene composite. Electrochem. Commun. 12, 303–306 (2010).
Xiang, H. et al. Graphene/nanosized silicon composites for lithium battery anodes with improved cycling stability. Carbon 49, 1787–1796 (2011).
Zhou, X., Yin, Y., Wan, L. & Guo, Y. Self-assembled nanocomposite of silicon nanoparticles encapsulated in graphene through electrostatic attraction for lithium-ion batteries. Adv. Energy Mater. 2, 1086–1090 (2012).
Luo, J. et al. Crumpled graphene-encapsulated Si nanoparticles for lithium ion battery anodes. J. Phys. Chem. Lett. 3, 1824–1829 (2012).
Lee, H., Dellatore, S. M., Miller, W. M. & Messersmith, P. B. Mussel-inspired surface chemistry for multifunctional coatings. Science 318, 426–430 (2007).
Liu, J. et al. Extension of the Stöber Method to the preparation of monodisperse resorcinol–formaldehyde resin polymer and carbon spheres. Angew. Chem. Int. Ed. 50, 5947–5951 (2011).
Li, N. et al. Sol–gel coating of inorganic nanostructures with resorcinol–formaldehyde resin. Chem. Commun. 49, 5135–5137 (2013).
Acknowledgements
Y.L. acknowledges the National Science Foundation Graduate Fellowship Program for funding and M. Hanna for fruitful discussions. H.-W.L. acknowledges the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (contract no. 2012038593). Y.C. acknowledges the support from the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy under the Battery Materials Research (BMR) Program.
Author information
Authors and Affiliations
Contributions
Y.L., K.Y. and Y.C. conceived and designed the experiments. Y.L. and K.Y. carried out materials synthesis and electrochemical characterization. Z.L. and N.L. participated in part of the synthesis and materials characterization. H.-W.L. and Y.L. conducted in situ TEM lithiation and electrical measurements. Y.L., K.Y. and Y.C. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1-13, Supplementary. (PDF 1406 kb)
Supplementary Video 1
External load testing for empty amorphous carbon shell. The fragile coating breaks after only a slight deformation. (MP4 1577 kb)
Supplementary Video 2
External load testing for empty graphene cage. Despite extreme compression, the graphene cage is able to fully collapse its shape and still returns to its original structure after deloading. (MP4 2429 kb)
Supplementary Video 3
Lithiation of a graphene-encapsulated SiMP. Note that the particle expansion and fracture are quite violent and anisotropic. Despite this, the graphene cage remains undamaged and electrically connects the fractured particles within. Movie is x10 speed. (MOV 9095 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Yan, K., Lee, HW. et al. Growth of conformal graphene cages on micrometre-sized silicon particles as stable battery anodes. Nat Energy 1, 15029 (2016). https://doi.org/10.1038/nenergy.2015.29
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nenergy.2015.29
This article is cited by
-
Progress and prospects of graphene-based materials in lithium batteries
Rare Metals (2024)
-
3D printed silicon-based micro-lattices with ultrahigh areal/gravimetric capacities and robust structural stability for lithium-ion batteries
Nano Research (2024)
-
Lithiated metallic molybdenum disulfide nanosheets for high-performance lithium–sulfur batteries
Nature Energy (2023)
-
Designing electrolytes and interphases for high-energy lithium batteries
Nature Reviews Chemistry (2023)
-
Realizing high-performance all-solid-state batteries with sulfide solid electrolyte and silicon anode: A review
Nano Research (2023)