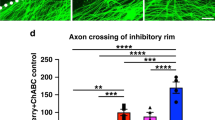
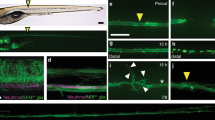
Abstract
In contrast to peripheral nerves, damaged axons in the mammalian brain and spinal cord rarely regenerate. Peripheral nerve injury stimulates neuronal expression of many genes that are not generally induced by CNS lesions, but it is not known which of these genes are required for regeneration. Here we show that co-expressing two major growth cone proteins, GAP-43 and CAP-23, can elicit long axon extension by adult dorsal root ganglion (DRG) neurons in vitro. Moreover, this expression triggers a 60-fold increase in regeneration of DRG axons in adult mice after spinal cord injury in vivo. Replacing key growth cone components, therefore, could be an effective way to stimulate regeneration of CNS axons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fidler, P. S. et al. Comparing astrocytic cell lines that are inhibitory or permissive for axon growth: the major axon-inhibitory proteoglycan is NG2. J. Neurosci. 19, 8778–8788 (1999).
Goldberg, J. L. & Barres, B. A. Nogo in nerve regeneration. Nature 403, 369–370 (2000).
Chen, M. S. et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403, 434–439 (2000).
Qiu, J., Cai, D. & Filbin, M. T. Glial inhibition of nerve regeneration in the mature mammalian CNS. Glia 29, 166–174 (2000).
Davies, S. J., Goucher, D. R., Doller, C. & Silver, J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J. Neurosci. 19, 5810–5822 (1999).
Skene, J. H. P. & Willard, M. Axonally transported proteins associated with axon growth in rabbit central and peripheral nervous systems. J. Cell Biol. 89, 96–103 (1981).
Skene, J. H. P. Axonal growth-associated proteins. Annu. Rev. Neurosci. 12, 127–156 (1989).
Caroni, P. Intrinsic neuronal determinants that promote axonal sprouting and elongation. Bioessays 19, 767–775 (1997).
Kalil, K. & Skene, J. H. P. Elevated synthesis of an axonally transported protein correlates with axon outgrowth in normal and injured pyramidal tracts. J. Neurosci. 6, 2563–2570 (1986).
Doster, S. K., Lozano, A. M., Aguayo, A. J. & Willard, M. B. Expression of the growth-associated protein GAP-43 in adult rat retinal ganglion cells following axon injury. Neuron 6, 1–13 (1991).
Schreyer, D. J. & Skene, J. H. P. Injury-associated induction of GAP-43 expression displays axon branch specificity in rat dorsal root ganglion neurons. J. Neurobiol. 24, 959–970 (1993).
Fernandes, K. J., Fan, D. P., Tsui, B. J., Cassar, S. L. & Tetzlaff, W. Influence of the axotomy to cell body distance in rat rubrospinal and spinal motoneurons: differential regulation of GAP-43, tubulins, and neurofilament-M. J. Comp. Neurol. 414, 495–510 (1999).
David, S. & Aguayo, A. J. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 214, 931–933 (1981).
Richardson, P. M., Issa, V. M. K. & Aguayo, A. J. Regeneration of long spinal axons in the rat. J. Neurocytol. 13, 165–182 (1984).
So, K.-F. & Aguayo, A. J. Lengthy regrowth of cut axons from ganglion cells after peripheral nerve transplantation into the retina of adult rats. Brain Res. 328, 349–354 (1985).
Friedman, B. & Aguayo, A. J. Injured neurons in the olfactory bulb of the adult rat grow axons along grafts of peripheral nerves. J. Neurosci. 5, 1616–1625 (1985).
Campbell, G., Anderson, P. N., Turmaine, M. & Lieberman, A. R. GAP-43 in the axons of mammalian CNS neurons regenerating into peripheral nerve grafts. Exp. Brain Res. 87, 67–74 (1991).
Schaden, H., Stuermer, C. A. O. & Bähr, M. GAP-43 immunoreactivity and axon regeneration in retinal ganglion cells of the rat. J. Neurobiol. 25, 1570–1578 (1994).
Chong, M. S. et al. GAP-43 expression in primary sensory neurons following central axotomy. J. Neurosci. 14, 4375–4384 (1994).
Richardson, P. M. & Issa, V. M. K. Peripheral injury enhances central regeneration of primary sensory neurons. Nature 309, 791–793 (1984).
Neumann, S. & Woolf, C. J. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 23, 83–91 (1999).
Meiri, K. F., Saffell, J. L., Walsh, F. S. & Doherty, P. Neurite outgrowth stimulated by neural cell adhesion molecules requires growth-associated protein-43 (GAP-43) function and is associated with GAP-43 phosphorylation in growth cones. J. Neurosci. 18, 10429–10437 (1998).
Aigner, L. & Caroni, P. Absence of persistent spreading, branching, and adhesion of GAP-43-depleted growth cones. J. Cell Biol. 128, 647–660 (1995).
Strittmatter, S. M., Fankhauser, C., Huang, P. L., Mashimo, H. & Fishman, M. C. Neuronal pathfinding is abnormal in mice lacking the neuronal growth cone protein GAP-43. Cell 80, 445–452 (1995).
Maier, D. L. et al. Disrupted cortical map and absence of cortical barrels in growth-associated protein (GAP)-43 knockout mice. Proc. Natl. Acad. Sci. USA 96, 9397–9402 (1999).
Zhu, Q. & Julien, J. P. A key role for GAP-43 in the retinotectal topographic organization. Exp. Neurol. 155, 228–242 (1999).
Caroni, P., Aigner, L. & Schneider, C. Intrinsic neuronal determinants locally regulate extrasynaptic and synaptic growth at the adult neuromuscular junction. J. Cell Biol. 136, 679–692 (1997).
Buffo, A. et al. Targeted overexpression of the neurite growth-associated protein B-50/GAP-43 in cerebellar Purkinje cells induces sprouting after axotomy but not axon regeneration into growth-permissive transplants. J. Neurosci. 17, 8778–8791 (1997).
Smith, D. S. & Skene, J. H. P. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J. Neurosci. 17, 646–658 (1997).
Aigner, L. et al. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell 83, 269–278 (1995).
Wiederkehr, A., Staple, J. & Caroni, P. The motility-associated proteins GAP-43, MARCKS, and CAP-23 share unique targeting and surface activity-inducing properties. Exp. Cell Res. 236, 103–116 (1997).
Mosevitsky, M. I. et al. The BASP1 family of myristoylated proteins abundant in axonal termini. Primary structure analysis and physico-chemical properties. Biochimie 79, 373–384 (1997).
Maekawa, S. et al. Cholesterol-dependent localization of NAP-22 on a neuronal membrane microdomain (raft). J. Biol. Chem. 274, 21369–21374 (1999).
Richardson, P. M. & Verge, V. M. K. The induction of a regenerative propensity in sensory neurons following peripheral axonal injury. J. Neurocytol. 15, 585–594 (1986).
Davies, S. J. et al. Regeneration of adult axons in white matter tracts of the central nervous system. Nature 390, 680–683 (1997).
Neve, R. L., Finch, E. A., Bird, E. D. & Benowitz, L. I. Growth-associated protein GAP-43 is expressed selectively in associative regions of the adult human brain. Proc. Natl. Acad. Sci. USA 85, 3638–3642 (1988).
Himi, T., Okazaki, T. & Mori, N. SCG10 mRNA localization in the hippocampus: comparison with other mRNAs encoding neuronal growth-associated proteins (nGAPs). Brain Res. 655, 177–185 (1994).
Sugiura, Y. & Mori, N. SCG10 expresses growth-associated manner in developing rat brain, but shows a different pattern to p19/stathmin or GAP-43. Dev. Brain Res. 90, 73–91 (1995).
McNamara, R. K. & Lenox, R. H. Comparative distribution of myristoylated alanine-rich C kinase substrate (MARCKS) and F1/GAP-43 gene expression in the adult rat brain. J. Comp. Neurol. 379, 48–71 (1997).
Higo, N., Oishi, T., Yamashita, A., Matsuda, K. & Hayashi, M. Quantitative non-radioactive in situ hybridization study of GAP-43 and SCG10 mRNAs in the cerebral cortex of adult and infant macaque monkeys. Cereb. Cortex 9, 317–331 (1999).
Frey, D., Laux, T., Xu, L., Schneider, C. & Caroni, P. Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outgrowth, and anatomical plasticity. J. Cell Biol. 149, 1443–1453 (2000).
Laux, T. et al. GAP43, MARCKS, and CAP23 modulate PI(4,5)P-2 at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J. Cell Biol. 149, 1455–1471 (1455).
Song, H. J. & Poo, M. -M. Signal transduction underlying growth cone guidance by diffusible factors. Curr. Opin. Neurobiol. 9, 355–363 (1999).
Mueller, B. K. Growth cone guidance: first steps towards a deeper understanding. Annu. Rev. Neurosci. 22, 351–388 (1999).
Li, Y., Field, P. M. & Raisman, G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science 277, 2000–2002 (1997).
Ramon-Cueto, A., Cordero, M. I., Santos-Benito, F. F. & Avila, J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron 25, 425–435 (2000).
McDonald, J. W. et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat. Med. 5, 1410–1412 (1999).
Huang, D. W., McKerracher, L., Braun, P. E. & David, S. A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron 24, 639–647 (1999).
Thallmair, M. et al. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nat. Neurosci. 1, 124–131 (1998).
Acknowledgements
We thank R. Chesebro for technical support, N. Cant for advice and assistance with retrograde labeling and L. Katz and J. Crowley for help with the fluorescence imaging. We also thank B. Finch, L. Katz, D. Purves, D. Chikaraishi, M. Nicolelis and A. Udvadia for comments on the manuscript. Support for this work was provided by the National Eye Institute (NIH), Novartis Pharmaceuticals and the Christopher Reeves Paralysis Foundation. K.R.B. is the recipient of a Ruth K. Broad Foundation fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bomze, H., Bulsara, K., Iskandar, B. et al. Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat Neurosci 4, 38–43 (2001). https://doi.org/10.1038/82881
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/82881
This article is cited by
-
Identification of potential biomarkers for ankylosing spondylitis based on bioinformatics analysis
BMC Musculoskeletal Disorders (2023)
-
Enhancing structural plasticity of PC12 neurons during differentiation and neurite regeneration with a catalytically inactive mutant version of the zRICH protein
BMC Neuroscience (2023)
-
Purified regenerating retinal neurons reveal regulatory role of DNA methylation-mediated Na+/K+-ATPase in murine axon regeneration
Communications Biology (2023)
-
Single cell atlas of spinal cord injury in mice reveals a pro-regenerative signature in spinocerebellar neurons
Nature Communications (2022)
-
Regeneration des Sehnerven – Wird das einmal Realität?
Die Ophthalmologie (2022)