Abstract
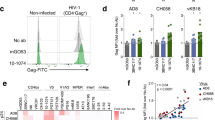
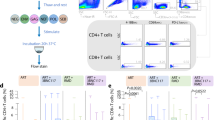
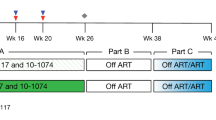
The concentration of human immunodeficiency virus type 1 (HIV–1) particles in blood plasma is very predictive of the subsequent disease course in an infected individual; its measurement has become one of the most important parameters for monitoring clinical status1. Steady–state virus levels in plasma reflect a balance between the rates of virions entering and leaving the peripheral blood2. We analyzed the rate of virus clearance in the general circulation in rhesus macaques receiving a continuous infusion of cell–free particles in the presence and absence of virus–specific antibodies. Here we show, by measuring virion RNA, particle–associated p24 Gag protein and virus infectivity, that the clearance of physical and infectious particles from a primary, dual–tropic virus isolate, HIV–1DH12, is very rapid in naive animals, with half–lives ranging from 13 to 26 minutes. In the presence of high–titer HIV–1DH12–specific neutralizing antibodies, the half–life of virion RNA was considerably reduced (to 3.9–7.2 minutes), and infectious virus in the blood became undetectable. Although physical virus particles were eliminated extravascularly, the loss of virus infectivity in the blood reflected the combined effects of extravascular clearance and intravascular inactivation of HIV–1 infectivity due to antibody binding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mellors, J.W. et al. Prognosis in HIV–1 predicted by the quantity of virus in plasma. Science 272, 1167– 1170 (1996).
Nathanson N. and Harrington B. Experimental Infection of Monkeys with Langat Virus. II. Turnover of Circulating Virus. Am. J. Epidemiol. 85, 494–502 (1967).
Shibata, R. et al. Generation of a chimeric human and simian immunodeficiency virus infectious to monkey peripheral blood mononuclear cells. J. Virol. 65, 3514–3520 (1991).
Shibata, R., Sakai, H., Kawamura, M., Tokunaga, K. & Adachi, A. Early replication block of human immunodeficiency virus type 1 in monkey cells. J. Gen. Virol. 76, 2723–2730 (1995).
Himathongkham, S. & Luciw, P.A. Restriction of HIV–1 (Subtype B) replication at the early step in rhesus macaque cells. Virology 219, 485–488 (1996).
Igarashi, T. et al. Protection of monkeys vaccinated with vpr and/or nef–defective simian immunodeficiency virus strain mac/human immunodeficiency virus type 1 chimeric viruses: a potential candidate live–attenuated human AIDS vaccine. J. Gen. Virol. 78, 985– 989 (1997).
Agy, M.B. et al. Infection of Macaca nemestrina by human immunodeficiency virus type–1. Science 103, 103– 106 (1992).
Shibata, R. et al. Isolation and characterization of a syncytium–inducing, macrophage/T–cell line–tropic human immunodeficiency virus type 1 isolate that readily infects chimpanzee cells in vitro and in vivo. J. Virol. 69, 4453– 4462 (1995).
Shibata, R. et al. Infection and Pathogenicity of Chimeric SIV/HIV Viruses in Macaques; Determinants of High Virus Loads and CD4 cell Killing. J. Infect. Dis. 176, 362–373 (1997).
Li, J., Lord, C.I., Haseltine, W., Letvin, N.L. & Sodroski, J. Infection of cynomolgus monkeys with a chimeric HIV–1/SIVmac virus that expresses the HIV–1 envelope glycoproteins. J. AIDS. 5, 639– 646 (1992).
Zilversmit, D.B., Boyd, G.A. & Brucer, M. The effect of particle size on blood clearance and tissue distribution of radioactive gold colloids. J. Lab. Clin. Med. 40, 255–266 (1952).
Shibata, R. et al. Resistance of previously infected chimpanzees to successive challenges with a heterologous intraclade B strain of human immunodeficiency virus type 1. J. Virol. 70, 4361– 4369 (1996).
Brunner, K.T., Hurez, D., McCluskey, R.T. & Benacerraf, B. Blood clearance of P32–labeled vesicular stomatitis and Newcastle disease viruses by the reticuloendothelial system in mice. J. Immunol. 85, 99–105 (1960).
Mims, C.A. Rift valley fever virus in mice. II. Adsorption and multiplication of virus. Brit. J. Exp. Pathol. 37, 110– 119 (1956).
Mims, C.A. The response of mice to large intravenous injections of Ectromelia virus II. The growth of virus in the liver. Brit. J. Exp. Pathol. 40, 533–542 (1959).
Schultz, I. & Neva, F.A. Relationship between blood clearance and viruria after intravenous injection of mice and rats with bacteriophage and poliovirus. J. Immunol. 94, 833– 841 (1965).
Wei, X. et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373, 117–122 (1995).
Ho, D. et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV–1 infection. Nature 373, 123– 126 (1995).
Perelson, A. et al. HIV–1 Dynamics in vivo: Virion clearance rate, infected cell life–span, and viral generation time. Science 271, 1582–1586 (1996).
National Institutes of Health in Guide for the Care and Use of Laboratory Animals, revised ed. DHHS publication number NIH 85–23 (1985).
Naidu Y.M. et al. Characterization of infectious molecular clones of simian immunodeficiency virus (SIVmac) and human immunodeficiency virus type 2: persistent infection of rhesus monkeys with molecularly cloned SIVmac. J. Virol. 62, 4691–4696 (1988).
Adachi A. et al. Production of acquired immunodeficiency syndrome–associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59, 284– 291(1986).
Shibata, R. et al. Reactivation of HIV Type 1 in chronically infected chimpanzees following xenostimulation with human cells or with pulses of corticosteroid. Aids Res. Hum. Retroviruses. 13, 377– 381 (1997).
Haigwood, N.L. et al. Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol. Lett. 51, 107–114 (1996).
Shibata, R., Siemon, C., Czajak, S.C., Desrosiers, R.C. & Martin, M.A. Live attenuated SIV vaccines elicit potent resistance against a challenge with an HIV–1 chimeric virus. J. Virol. 71, 8141–8148 (1997).
Willey R.L. et al. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J. Virol. 62, 139–147 (1988).
Reed, L.J. & Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27, 493–497 (1938).
Dewar, R.L. et. al. Application of branched DNA signal amplification to monitor human immunodeficiency virus type 1 burden in human plasma. J. Infect. Dis. 170, 1172–1179 (1994).
Acknowledgements
We thank G. Coleman for assistance in the animal experiments; M. Eckhaus and G. Miller for pathological analyses; C. Pierce for purification of immunoglobulin; M.G. Lewis for providing SHIV HXB2 infected monkeys; and R. Dewar for bDNA assays.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Igarashi, T., Brown, C., Azadegan, A. et al. Human immunodeficiency virus type 1 neutralizing antibodies accelerate clearance of cell–free virions from blood plasma. Nat Med 5, 211–216 (1999). https://doi.org/10.1038/5576
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/5576
This article is cited by
-
Monoklonale Antikörper zur antiinfektiven Therapie
Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (2020)
-
Early antibody therapy can induce long-lasting immunity to SHIV
Nature (2017)
-
Mathematical model of multivalent virus–antibody complex formation in humans following acute and chronic HIV infections
Journal of Mathematical Biology (2015)
-
Cell-associated HIV RNA: a dynamic biomarker of viral persistence
Retrovirology (2013)
-
Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia
Nature (2013)