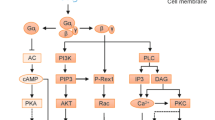
Abstract
Inappropriate or prolonged inflammation is the main cause of many diseases1; for this reason it is important to understand the physiological mechanisms that terminate inflammation in vivo2. Agonists for several Gs-protein-coupled receptors3, including cell-surface adenosine purinergic receptors4,5,6,7, can increase levels of immunosuppressive cyclic AMP in immune cells8,9,10,11,12,13,14,15; however, it was unknown whether any of these receptors regulates inflammation in vivo. Here we show that A2a adenosine receptors have a non-redundant role in the attenuation of inflammation and tissue damage in vivo. Sub-threshold doses of an inflammatory stimulus16,17 that caused minimal tissue damage in wild-type mice were sufficient to induce extensive tissue damage, more prolonged and higher levels of pro-inflammatory cytokines, and death of male animals deficient in the A2a adenosine receptor. Similar observations were made in studies of three different models of inflammation and liver damage as well as during bacterial endotoxin-induced septic shock. We suggest that A2a adenosine receptors are a critical part of the physiological negative feedback mechanism for limitation and termination of both tissue-specific and systemic inflammatory responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tarkowski, A. & Wagner, H. Arthritis and sepsis caused by Staphylococcus aureus: can the tissue injury be reduced by modulating the host's immune system? Mol. Med. Today 4, 15–18 (1998).
Levy, B. D., Clish, C. B., Schmidt, B., Gronert, K. & Serhan, C. N. Lipid mediator class switching during acute inflammation: signals in resolution. Nature Immunol. 2, 612–619 (2001).
Gilman, A. G. G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 56, 615–649 (1987).
Olah, M. E. & Stiles, G. L. Adenosine receptor subtypes: characterization and therapeutic regulation. Annu. Rev. Pharmacol. Toxicol. 35, 581–606 (1995).
Fredholm, B. B. et al. Structure and function of adenosine receptors and their genes. Naunyn-Schmiedeberg's Arch. Pharmacol. 362, 364–374 (2000).
Burnstock, G. Purine-mediated signaling in pain and visceral perception. Trends Pharmacol. Sci. 22, 182–188 (2001).
Linden, J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu. Rev. Pharmacol. Toxicol. 41, 775–787 (2001).
Cronstein, B. N. Adenosine, an endogenous anti-inflammatory agent. J. Appl. Physiol. 76, 5–13 (1994).
Cronstein, B. N. A novel approach to the development of anti-inflammatory agents: adenosine release at the inflamed sites. J. Invest. Med. 43, 50–57 (1995).
Firestein, G. S. et al. Protective effect of an adenosine kinase inhibitor in septic shock. J. Immunol. 152, 5853–5859 (1994).
Huang, S., Koshiba, M., Apasov, S. & Sitkovsky, M. Role of A2a adenosine receptor-mediated signaling in inhibition of T cell activation and expansion. Blood 90, 1600–1610 (1997).
van der Pouw Kraan, T. C. T. M. et al. Histamine inhibits the production of interleukin-12 through interaction with H2 receptors. J. Clin. Invest. 102, 1866–1873 (1998).
Malfait, A.-M. et al. The β2-adrenergic agonist salbutamol is a potent suppressor of established collagen-induced arthritis: mechanisms of action. J. Immunol. 162, 6278–6283 (1999).
Eigler, A. et al. Endogenous adenosine curtails lipopolysaccharide-stimulated tumour necrosis factor synthesis. Scand. J. Immunol. 45, 132–139 (1997).
Sullivan, G. W., Sarembock, I. J. & Linden, J. The role of inflammation in vascular diseases. J. Leukoc. Biol. 67, 591–602 (2000).
Tiegs, G. Experimental hepatitis and role of cytokines. Acta Gastroenterol. Belg. 60, 176–179 (1997).
Kaneko, Y. et al. Augmentation of Valpha14 NK T cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J. Exp. Med. 191, 105–114 (2000).
Winn, H. R., Rubio, R. & Berne, R. M. Brain adenosine concentration during hypoxia in rat. Am. J. Physiol. 241, H235–H242 (1981).
Van Belle, H., Goossens, F. & Wynants, J. Formation and release of purine catabolites during hypoperfusion, anoxia, and ischemia. Am. J. Pathol. 252, H886–H893 (1987).
Rudolphi, K. A., Schubert, P., Parkinson, F. E. & Fredholm, B. B. Neuroprotective role of adenosine in cerebral ischemia. Trends Pharmacol. Sci. 13, 439–445 (1992).
Marquardt, D. L., Gruber, H. E. & Wasserman, S. I. Adenosine release from stimulated mast-cells. Proc. Natl Acad. Sci. USA 81, 6192–6196 (1984).
Filippini, A., Taffs, R. E. & Sitkovsky, M. V. Extracellular ATP in T-lymphocyte activation: possible role in effector functions. Proc. Natl Acad. Sci. USA 87, 8267–8271 (1990).
Hoskin, D. W., Reynolds, T. & Blay, J. Adenosine as a possible inhibitor of killer T-cell activation in the microenvironment of solid tumours. Int. J. Cancer 59, 854–855 (1994).
Ledent, C. et al. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388, 674–678 (1997).
Chen, J.-F. et al. A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J. Neurosci. 19, 9192–9200 (1999).
Apasov, S. G. et al. Study of A2A adenosine receptor gene deficient mice reveals that adenosine analogue CGS 21680 possesses no A2A receptor-unrelated lymphotoxicity. Br. J. Pharmacol. 131, 43–50 (2000).
Armstrong, J. M. et al. Gene dose effect reveals no Gs protein coupled A2A adenosine receptor reserve in murine T lymphocytes. Studies of cells from A2A receptor gene-deficient mice. J. Biochem. 354, 123–130 (2001).
Ji, X.-D. & Jacobson, K. A. Use of the triazolotriazine [3H]ZM 241385 as a radioligand at recombinant human A2B adenosine receptors. Drug Design Disc. 16, 217–226 (1999).
Shumann, J., Angermuller, S., Bang, R., Lohoff, M. & Tiegs, G. Acute hepatotoxicity of Pseudomonas aeruginosa exotoxin A in mice depends on T cells and TNF. J. Immunol. 161, 5745–5754 (1998).
Luster, M. I. et al. Role of inflammation in chemical-induced hepatotoxicity. Toxicol. Lett. 120, 317–321 (2001).
Acknowledgements
We thank J. Fan Chen, S. Fink and M. A. Schwartzschild for providing A2a receptor gene deficient mice; W. E. Paul, R. N. Germain, S. Apasov, P. Smith and P. M. Murphy for support, discussions and help; and J. Kinsel and S. Starnes for help in preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Ohta, A., Sitkovsky, M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414, 916–920 (2001). https://doi.org/10.1038/414916a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/414916a
This article is cited by
-
The effect of extracorporeal shock wave on joint capsule fibrosis based on A2AR-Nrf2/HO-1 pathway in a rat extending knee immobilization model
Journal of Orthopaedic Surgery and Research (2023)
-
Caffeine-folic acid-loaded-chitosan nanoparticles combined with methotrexate as a novel HepG2 immunotherapy targeting adenosine A2A receptor downstream cascade
BMC Complementary Medicine and Therapies (2023)
-
Purinergic signaling during Marek’s disease in chickens
Scientific Reports (2023)
-
Targeting hypoxia-inducible factors: therapeutic opportunities and challenges
Nature Reviews Drug Discovery (2023)
-
Inhibition of lipopolysaccharide-induced inflammation by trophoblast-conditioned medium and trophoblast-derived extracellular vesicles in human middle ear epithelial cells
Scientific Reports (2023)