Abstract
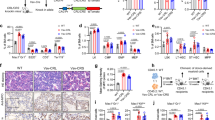
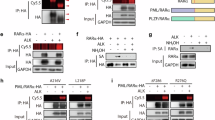
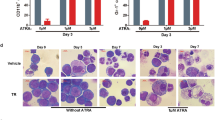
The transforming proteins of acute promyelocytic leukaemias (APL) are fusions of the promyelocytic leukaemia (PML) and the promyelocytic leukaemia zinc-finger (PLZF) proteins with retinoic acid receptor-α (RARα)1,2. These proteins retain the RARα DNA- and retinoic acid (RA)-binding domains, and their ability to block haematopoietic differentiation depends on the RARα DNA-binding domain3,4,5,6. Thus RA-target genes are downstream effectors7,8. However, treatment with RA induces differentiation of leukaemic blast cells and disease remission in PML–RARα APLs, whereas PLZF–RARα APLs are resistant to RA1,2. Transcriptional regulation by RARs involves modifications of chromatin by histone deacetylases, which are recruited to RA-target genes by nuclear co-repressors9,10. Here we show that both PML–RARα and PLZF–RARα fusion proteins recruit the nuclear co-repressor (N-CoR)–histone deacetylase complex through the RARα CoR box. PLZF–RARα contains a second, RA-resistant binding site in the PLZF amino-terminal region. High doses of RA release histone deacetylase activity from PML–RARα, but not from PLZF–RARα. Mutation of the N-CoR binding site abolishes the ability of PML–RARα to block differentiation, whereas inhibition of histone deacetylase activity switches the transcriptional and biological effects of PLZF–RARα from being an inhibitor to an activator of the RA signalling pathway. Therefore, recruitment of histone deacetylase is crucial to the transforming potential of APL fusion proteins, and the different effects of RA on the stability of the PML–RARα and PLZF–RARα co-repressor complexes determines the differential response of APLs to RA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Grignani, F. et al. Acute promyelocytic leukaemia: from genetics to treatment. Blood 83, 10–25 (1994).
Warrell, R. P. J., de Thé, H., Wang, Z. Y. & Degos, L. Acute promyelocytic leukaemia. N. Engl. J. Med. 324, 177–189 (1993).
de The, H. et al. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell 66, 675–684 (1991).
Kakizuka, A. et al. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell 66, 663–674 (1991).
Pandolfi, P. P. et al. Genomic variability and alternative splicing generate multiple PML/RAR alpha transcripts that encode aberrant PML proteins and PML/RAR alpha isoforms in acute promyelocytic leukaemia. EMBO J. 11, 1397–1407 (1992).
Chen, Z. et al. Fusion between a novel Krüppel-like zinc finger gene and retinoic acid receptor-α locus due to a variant t(11;17) translocation associated with acute promyelocytic leukaemia. EMBO J. 12, 1161–1167 (1993).
Chen, Z. et al. PLZF-RAR alpha fusion proteins generated from the variant t(11;17)(q23;q21) translocation in acute promyelocytic leukemia inhibit ligand-dependent transactivation of wild-type retinoic acid recetors. Proc. Natl Acad. Sci. USA 91, 1178–1182 (1994).
Ruthardt, M. et al. Opposite effects of the Acute Promyelocytic Leukaemia PML/RARα and PLZF/RARα fusion proteins on retinoic acid signalling. Mol. Cell. Biol. 17, 4859–4869 (1997).
Heinzel, T. et al. Acomplex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature 387, 43–49 (1997).
Nagy, L. et al. Nuclear receptor repression mediated by a complex containing SMRT,,mSin3A, and histone deacetylase. Cell 89, 373–380 (1997).
Grunstein, M. Histone acetylation in chromatin structure and transcription. Nature 389, 349–352 (1997).
Wolffe, A. P. Sinful repression. Nature 387, 16–17 (1997).
Mangelsdorf, D. J. & Evans, R. M. The RXR heterodimers and orphan receptors. Cell 83, 841–850 (1995).
Minucci, S. & Ozato, K. Retinoid receptors in transcriptional regulation. Curr. Opin. Genet. Dev. 6, 567–574 (1996).
Chambon, P. Adecade of molecular biology of retinoid receptors. FASEB J. 10, 940–954 (1996).
Hong, S., David, G., Wong, C., Dejean, A. & Privalsky, M. SMRT corepressor interacts with PLZF and with the PML-retinoic acid receptor alpha and PLZF-RAR alpha oncoproteins associated with acute promyelocytic leukaemia. Proc. Natl Acad. Sci. USA 94, 9028–9033 (1997).
Grignani, F. et al. The acute promyelocytic leukemia-specific PML-RAR alpha fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. Cell 74, 423–431 (1993).
Grignani, F. et al. Effects on differentiation by the promyelocytic leukemia PML/RARalpha protein depend on the fusion of the PML protein dimerization and RARalpha DNA binding domains. EMBO J. 15, 4949–4958 (1996).
Horlein, A. J. et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377, 397–403 (1995).
Dhordain, P. et al. SMRT binds the BTB/POZ repressing domain of the LAZ3/BCL6 oncoprotein. Proc. Natl Acad. Sci. USA 94, 10727–10767 (1997).
Yoshida, M., Kijima, M., Akita, M. & Beppu, T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem. 265, 17174–17179 (1990).
Nagy, L. et al. Identification and characterization of a versatile retinoid response element in the mouse tissue transglutaminase gene promoter. J. Biol. Chem. 271, 4355–4365 (1996).
Benedetti, L. et al. Retinoic-induced differentiation of acute promyelocytic leukemia involves PML-RARα-mediated increase of type II transglutaminase. Blood 87, 1939–1950 (1996).
Borrow, J. et al. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the REB-binding protein. Nature Genet. 14, 33–41 (1996).
Zamir, I. et al. Anuclear hormone receptor coreceptor mediates transcriptional silencing by receptors with distinct repression domains. Mol. Cell. Biol. 16, 5458–5465 (1996).
Nervi, C. et al. Characterisation of the PML/RAR chimeric product of the APL-specific t(15;17) translocation. Cancer Res. 52, 3687–3692 (1992).
Bartl, S. et al. Identification of mouse histone deacetylase I as a growth-factor inducible gene. Mol. Cell. Biol. 17, 5033–5043 (1997).
Sundstrom, C. & Nilsson, K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int. J. Cancer 17, 565–577 (1976).
Lin, R. J. et al. Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature 391, 811–814 (1998).
Acknowledgements
We thank P. P. DiFiore, K. Ozato, K. Helin, T. Casini and M. Maccarana for discussions, and C. Matteucci, S. DiPietro and S. Lupo for technical help. S.D.M., V.G. and M.F. are recipients of fellowships from A.U.L.L., A.I.R.C. and INT (Milan), respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grignani, F., De Matteis, S., Nervi, C. et al. Fusion proteins of the retinoic acid receptor-α recruit histone deacetylase in promyelocytic leukaemia. Nature 391, 815–818 (1998). https://doi.org/10.1038/35901
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35901