Abstract
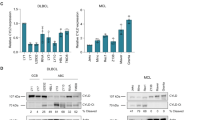
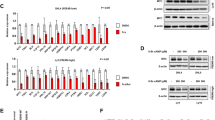
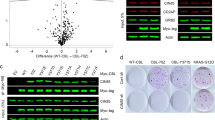
Burkitt's lymphoma (BL) is a highly malignant B-cell tumour characterized by chromosomal translocations that constitutively activate the c-myc oncogene. Here we show that BL cells are resistant to apoptosis and do not accumulate ubiquitin conjugates in response to otherwise toxic doses of inhibitors of the proteasome. Deubiquitinating enzymes and the cytosolic subtilisin-like protease tripeptidylpeptidase II are upregulated in BLs, and could be rapidly induced by the overexpression of c-myc in normal B cells carrying oestrogen-driven recombinant Epstein–Barr virus. Apoptosis was induced by inhibiting tripeptidylpeptidase II, suggesting that the activity of this protease may be required for the survival of BL cells. We thus show that there is a regulatory link between c-myc activation and changes in proteolysis that may affect malignant transformation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ciechanover, A. The proteasome-ubiquitin pathway: on protein death and cell life. EMBO J ensates for loss of proteasome function. Nature 392, 618–622 (1998).
Baumeister, W., Walz, J., Zühl, F. & Seemüller, E. The proteasome: paradigm of a self-compartmentalizing protease. Cell 92, 367–380 (1998).
Sherr, C. J. Cancer cell cycles. Science 274, 1672–1677 (1996).
Geier, E. et al. A giant protease with potential to substitute for some functions of the proteasome. Science 283, 978–981 (1999).
Glas, R., Bogyo, M., McMaster, J., Gaczynska, M. & Ploegh, H. A proteolytic system that compensates for loss of proteasome function. Nature 392, 618–622 (1998).
Klein, G. Specific chromosomal translocations in the genesis of B-cell lymphomas in mice and men. Cell 32, 311–315 (1983).
Rowe, M. et al. Differences in B cell growth phenotype reflect novel patterns of Epstein–Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 6, 2743–2751 (1987).
Masucci, M. G. et al. Down-regulation of class I HLA antigens and of the Epstein–Barr virus-encoded latent membrane protein in Burkitt lymphoma lines. Proc. Natl Acad. Sci. USA 84, 4567–4571 (1987).
Frisan, T., Levitsky, V., Polack, A. & Masucci, M. Phenotype-dependent differences in proteasome subunit composition and cleavage specificity in B cell lines. J. Immunol. 160, 3281–3289 (1998).
Frisan, T., Levitsly, V. & Masucci, M. G. Variations in proteasome subunit composition and enzymatic activity in B-lymphoma lines and normal B-cells. Int. J. Cancer 88, 881–888 (2000).
Rowe, M. et al. Restoration of endogenous antigen processing in Burkitt's lymphoma cells by Epstein–Barr virus latent membrane protein-1: coordinate up-regulation of peptide transporters and HLA-class I antigen expression. Eur. J. Immunol. 25, 1374–1384 (1995).
Kempkes, B. et al. B-cell proliferation and induction of early G1-regulating proteins by Epstein–Barr virus mutants conditional for EBNA2. EMBO J. 14, 88– (1995).
Polack, A. et al. c-Myc activation renders proliferation of Epstein–Barr virus (EBV)-transformed cells independent of EBV nuclear antigen 2 and latent membrane protein 1. Proc. Natl Acad. Sci. USA 93 (1996).
Pajic, A. et al. Cell cycle activation by c-myc in a Burkitt's lymphoma model cell line. Int. J. Cancer 87, 788–793 (2000).
Hochstrasser, M. Ubiquitin-dependent protein degradation. Annu. Rev. Genet. 30, 405–439 (1996).
Hadari, T., Warms, J. V. B., Rose, I. A. & Hersko, A. A ubiquitin C-terminal isopeptidase that acts on polyubiquitin chains. J. Biol. Chem. 267, 719–727 (1992).
Papa, F. & Hochstrasser, M. The yeast DOA4 gene encodes a deubiquitinating enzyme related to the product of the tre-2 oncogene. Nature 366, 313–319 (1993).
Huang, Y., Backer, T. T. & Fisher-Vize, J. A. Control of cell fate by a deubiquitinating enzyme encoded by the fat facet gene. Science 270, 1828–1831 (1995).
Zhu, Y., Carroll, M., Papa, F. R., Hochstrasser, M. & D'Andrea, A. D. DUB-1, a novel deubiquitinating enzyme with growth-suppressing activity. Proc. Natl Acad. Sci. USA 93, 3275–3279 (1996).
Rorth, P., Szabo, K. & Texido, G. The level of C/EBP protein is critical for cell migration during drosophila oogenesis and is tightly controlled by regulated degradation. Mol. Cell 6, 23–30 (2000).
Bålöw, R.-M., Tomkinson, B., Ragnarsson, U. & Zetterqvist . Purification, substrate specificity, and classification of tripeptidyl peptidase II. J. Biol. Chem. 261, 2409–2417 (1986).
Rose, C. et al. Characterisation and inhibition of a cholecystokinin-inactivating serine peptidase. Nature 380, 403–409 (1986).
Henriksson, M. & Luscher, B. Proteins of the Myc network: essential regulators of cell growth and differentiation. Adv. Cancer Res. 68, 109–182 (1996).
Marku, C. G., Bossone, S. A. & Patel, A. J. Myc function and regulation. Annu. Rev. Biochem. 61, 806–860 (1992).
Gross-Mesilaty, S. et al. Basal and human papillomavirus E6 oncoprotein-induced degradation of Myc proteins by the ubiquitin pathway. Proc. Natl Acad. Sci. USA 95, 8058–8063 (1998).
Salghetti, S. E., Kim, S. Y. & Tansey, W. P. Destruction of Myc by ubiquitin-mediated proteolysis: cancer associated and transforming mutations stabilize Myc. EMBO J. 18, 717–726 (1999).
Gregory, M. A. & Hann, S. R. c-Myc proteolysis by the ubiquitin-proteasome pathway: stabilization of c-Myc in Burkitt's lymphoma cells. Mol. Cell. Biol. 20, 2423–2435 (2000).
Bathia, K. et al. Point mutations in the c-Myc transactivation domain are common in Burkitt's lymphoma and mouse plasmocytoma. Nature Genet. 5, 56–61 (1993).
Miller, G. & Lipman, M. Release of infectious Epstein–Barr virus by transformed marmoset leukocytes. Proc. Natl Acad. Sci. USA 70, 190–194 (1973).
Diabata, M., Humphreys, R. E., Takada, K. & Sairenji, T. Activation of latent EBV via anti-IgG-triggered, second messenger pathways in the Burkitt's lymphoma cell line Akata. J. Immunol. 144, 4788–4793 (1990).
Klein, G., Dombos, L. & Gothoskar, B. Sensitivity of Epstein–Barr virus (EBV) producer and non-producer human lymphoblastoid cell lines to superinfection with EB-virus. Int. J. Cancer 10, 44–57 (1972).
Rooney, C. M. et al. Endemic Burkitt's lymphoma: Phenotypic analysis of tumour biopsy cells and of the derived tumour cell lines. J. Natl Cancer Inst. 77, 681–687 (1986).
Farvot, M. C. et al. Distinct reactivity of Burkitt's lymphoma cell lines with eight monoclonal antibodies correlated with ethnic origin. J. Natl Cancer Inst. 73, 841–847 (1984).
Drexler, H. C. A. Activation of the cell death program by inhibition of proteasome function. Proc. Natl Acad. Sci. USA 94, 855–860 (1997).
Gaczynska, M., Rock, K. L. & Goldberg, A. L. Gamma-interferon and expression of MHC genes regulate peptide hydrolysis by proteasomes. Nature 365, 264–267 (1993).
Dang, L. C., Melandri, F. D. & Stein, R. L. Kinetic and mechanistic studies on the hydrolysis of ubiquitin C-terminal 7-amido-4-methylcoumarin by deubiquitinating enzymes. Biochemistry 37, 1868–1879 (1998).
Rock, K. L. et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78, 761–771 (1994).
Driscoll, J., Brown, M. G., Finley, D. & Monaco, J. J. MHC-linked LMP gene products specifically alter peptidase activities of the proteasome. Nature 365, 262–264 (1993).
Acknowledgements
We thank H. Ploegh and B. Tomkinson for the NLVS and butabindide, and members of the Masucci laboratory for helpful discussions and critically reading the manuscript. This work was supported by grants from the Swedish Cancer Society, the Swedish Foundation of Strategy Research and the Petrus and Augusta Hedlund Foundation, Stockholm, Sweden, and by the DFG. S.V. was supported by a fellowship from the European Commission Training and Mobility Program on `The central role of the ubiquitin proteasome system in regulatory processes involved in immunological, inflammatory, endocrinological and malignant disorders'.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gavioli, R., Frisan, T., Vertuani, S. et al. c-myc overexpression activates alternative pathways for intracellular proteolysis in lymphoma cells. Nat Cell Biol 3, 283–288 (2001). https://doi.org/10.1038/35060076
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35060076
This article is cited by
-
A cysteine near the C-terminus of UCH-L1 is dispensable for catalytic activity but is required to promote AKT phosphorylation, eIF4F assembly, and malignant B-cell survival
Cell Death Discovery (2019)
-
The protein-interaction network with functional roles in tumorigenesis, neurodegeneration, and aging
Molecular and Cellular Biochemistry (2016)
-
Novel protein–protein interactions of TPPII, p53, and SIRT7
Molecular and Cellular Biochemistry (2015)
-
The de-ubiquitinase UCH-L1 is an oncogene that drives the development of lymphoma in vivo by deregulating PHLPP1 and Akt signaling
Leukemia (2010)
-
c-Myc overexpression promotes a germinal center-like program in Burkitt's lymphoma
Oncogene (2010)