Abstract
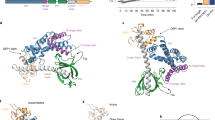
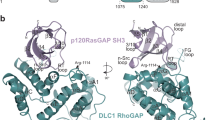
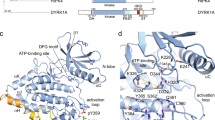
The principal guanine nucleotide exchange factors for Rho family G proteins contain tandem Dbl-homology (DH) and pleckstrin-homology (PH) domains that catalyse nucleotide exchange and the activation of G proteins. Here we have determined the crystal structure of the DH and PH domains of the T-lymphoma invasion and metastasis factor 1 (Tiam1) protein in complex with its cognate Rho family G protein, Rac1. The two switch regions of Rac1 are stabilized in conformations that disrupt both magnesium binding and guanine nucleotide interaction. The resulting cleft in Rac1 is devoid of nucleotide and highly exposed to solvent. The PH domain of Tiam1 does not contact Rac1, and the position and orientation of the PH domain is markedly altered relative to the structure of the uncomplexed, GTPase-free DH/PH element from Sos1. The Tiam1/Rac1 structure highlights the interactions that catalyse nucleotide exchange on Rho family G proteins, and illustrates structural determinants dictating specificity between individual Rho family members and their associated Dbl-related guanine nucleotide exchange factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cerione, R. A. & Zheng, Y. The Dbl family of oncogenes. Curr. Opin. Cell Biol. 8, 216–222 (1996).
Whitehead, I. P., Campbell, S., Rossman, K. L. & Der, C. J. Dbl family proteins. Biochim. Biophys. Acta 1332, F1–F23 (1997).
Van Aelst, L. & D'Souza-Schorey, C. Rho GTPases and signaling networks. Genes Dev. 11, 2295– 2322 (1997).
Mackay, D. J. & Hall, A. Rho GTPases. J. Biol. Chem. 273, 20685–20688 ( 1998).
Khosravi-Far, R., Campbell, S., Rossman, K. L. & Der, C. J. Increasing complexity of Ras signal transduction: involvement of Rho family proteins. Adv. Cancer Res. 72, 57– 107 (1998).
Zohn, I. M., Campbell, S. L., Khosravi-Far, R., Rossman, K. L. & Der, C. J. Rho family proteins and Ras transformation: the RHOad less traveled gets congested. Oncogene 17 , 1415–1438 (1998).
Eva, A. & Aaronson, S. A. Isolation of a new human oncogene from a diffuse B-cell lymphoma. Nature 316, 273–275 (1985).
Zheng, Y., Cerione, R. & Bender, A. Control of the yeast bud-site assembly GTPase Cdc42. Catalysis of guanine nucleotide exchange by Cdc24 and stimulation of GTPase activity by Bem3. J. Biol. Chem. 269, 2369 –2372 (1994).
Hordijk, P. L. et al. Inhibition of invasion of epithelial cells by Tiam1–Rac signaling. Science 278, 1464– 1466 (1997).
Michiels, F., Habets, G. G., Stam, J. C., van der Kammen, R. A. & Collard, J. G. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature 375, 338–340 (1995).
Han, J. et al. Lck regulates Vav activation of members of the Rho family of GTPases. Mol. Cell Biol. 17, 1346– 1353 (1997).
Habets, G. G. et al. Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell 77, 537–549 (1994).
Habets, G. G., van der Kammen, R. A., Stam, J. C., Michiels, F. & Collard, J. G. Sequence of the human invasion-inducing TIAM1 gene, its conservation in evolution and its expression in tumor cell lines of different tissue origin. Oncogene 10, 1371–1376 (1995).
Stam, J. C. et al. Targeting of Tiam1 to the plasma membrane requires the cooperative function of the N-terminal pleckstrin homology domain and an adjacent protein interaction domain. J. Biol. Chem. 272, 28447–28454 (1997).
Soisson, S. M., Nimnual, A. S., Uy, M., Bar-Sagi, D. & Kuriyan, J. Crystal structure of the Dbl and pleckstrin homology domains from the human Son of sevenless protein. Cell 95, 259–268 (1998).
Collaborative Computational Project, Number 4. Acta Crystallogr. D 50, 760– 763 (1994).
Ferguson, K. M., Lemmon, M. A., Schlessinger, J. & Sigler, P. B. Structure of the high affinity complex of inositol trisphosphate with a phospholipase C pleckstrin homology domain. Cell 83, 1037 –1046 (1995).
Kubiseski, T. J., Chook, Y. M., Parris, W. E., Rozakis-Adcock, M. & Pawson, T. High affinity binding of the pleckstrin homology domain of mSos1 to phosphatidylinositol (4,5)-bisphosphate. J. Biol. Chem. 272, 1799–1804 (1997).
Anborgh, P. H. et al. Ras-specific exchange factor GRF: oligomerization through its Dbl homology domain and calcium-dependent activation of Raf. Mol. Cell Biol. 19, 4611–4622 (1999).
Aghazadeh, B. et al. Structure and mutagenesis of the Dbl homology domain. Nature Struct. Biol. 5, 1098–1107 (1998).
Liu, X. et al. NMR structure and mutagenesis of the N-terminal Dbl homology domain of the nucleotide exchange factor Trio. Cell 95, 269–277 (1998).
Blomberg, N. & Nilges, M. Functional diversity of PH domains: an exhaustive modelling study. Fold. Des. 2, 343–355 (1997).
Rebecchi, M. J. & Scarlata, S. Pleckstrin homology domains: a common fold with diverse functions. Annu. Rev. Biophys. Biomol. Struct. 27, 503–528 (1998).
Hirshberg, M., Stockley, R. W., Dodson, G. & Webb, M. R. The crystal structure of human rac1, a member of the rho-family complexed with a GTP analogue. Nature Struct. Biol. 4, 147–152 (1997).
Cherfils, J. & Chardin, P. GEFs: structural basis for their activation of small GTP-binding proteins. Trends Biochem. Sci. 24, 306–311 ( 1999).
Steven, R. et al. UNC-73 activates the Rac GTPase and is required for cell and growth cone migrations in C. elegans. Cell 92 , 785–795 (1998).
Kawashima, T., Berthet-Colominas, C., Wulff, M., Cusack, S. & Leberman, R. The structure of the Escherichia coli EF-Tu:EF-Ts complex at 2.5 Å resolution. Nature 379, 511–518 ( 1996).
Boriack-Sjodin, P. A., Margarit, S. M., Bar-Sagi, D. & Kuriyan, J. The structural basis of the activation of Ras by Sos. Nature 394, 337–343 (1998).
Goldberg, J. Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell 95, 237–248 (1998).
Shimizu, T. et al. An open conformation of switch I revealed by the crystal structure of a Mg2+-free form of RHOA complexed with GDP. Implications for the GDP/GTP exchange mechanism. J. Biol. Chem. 275, 18311–18317 (2000).
Leonard, D. et al. The identification and characterization of a GDP-dissociation inhibitor (GDI) for the CDC42Hs protein. J. Biol. Chem. 267, 22860–22868 (1992).
Hoffman, G. R., Nassar, N. & Cerione, R. A. Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell 100, 345–356 (2000).
Han, J. et al. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science 279, 558–560 (1998).
Lenzen, C., Cool, R. H. & Wittinghofer, A. Analysis of intrinsic and Cdc25-stimulated guanine nucleotide exchange of p21ras-nucleotide complexes by flourescence measurements. Methods Enzymol. 255, 95–109 (1995).
Otwinowski, Z. Data Collection and Processing (eds Sawyer, L., Isaacs, N. & Bailey, S.) (Daresbury Laboratory, Warrington, UK, 1993).
Sheldrick, G. M. SHELXS86 – Program for Crystal Structural Solution (Univ. Gottingen, Gottingen, Germany, 1986).
Otwinowski, Z. (ed.) Maximum Likelihood Refinement of Heavy Atom Parameters (Daresbury, Warrington, UK, 1991).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Brünger, A. T. et al. A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905– 921 (1998).
Kraulis, P. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946 –950 (1991).
Merritt, E. A. & Murphy, M. E. P. Raster3D Version 2.0. A program for photorealistic molecular graphics. Acta Crystallogr. D 50, 869–873 (1994).
Carson, M. Ribbons. Methods Enzymol. 277, 493– 505 (1997).
Nicholls, A., Sharp, K. A. & Honig, B. Protein folding and associations: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins Struct. Funct. Genet. 11, 281–296 (1991).
Acknowledgements
We would like to thank M. Pham and S. Foster for technical assistance; J. Snyder for assistance with data collection; L. Betts, H. Ke, C. Der, D. Siderovski, B. Worthylake, A. Singer, and C. Winkelman for critical reading of this manuscript; C. Ogata, and R. Abramowitz of beamline X4A, and the staff of NSLS for help with synchrotron data collection; D.K.W. is supported by a grant from the American Cancer Society, J.S. acknowledges support from the NIH and the Pew Charitable Trusts.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Worthylake, D., Rossman, K. & Sondek, J. Crystal structure of Rac1 in complex with the guanine nucleotide exchange region of Tiam1. Nature 408, 682–688 (2000). https://doi.org/10.1038/35047014
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35047014
This article is cited by
-
The polarity protein PARD3 and cancer
Oncogene (2021)
-
Targeting the cytoskeleton against metastatic dissemination
Cancer and Metastasis Reviews (2021)
-
Deep phylogeny of cancer drivers and compensatory mutations
Communications Biology (2020)
-
Vav2 pharmaco-mimetic mice reveal the therapeutic value and caveats of the catalytic inactivation of a Rho exchange factor
Oncogene (2020)
-
TIPE2 acts as a biomarker for tumor aggressiveness and suppresses cell invasiveness in papillary thyroid cancer (PTC)
Cell & Bioscience (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.