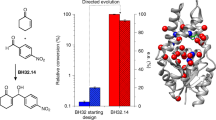
Abstract
In biological systems, enzymes catalyse the efficient synthesis of complex molecules under benign conditions, but widespread industrial use of these biocatalysts depends crucially on the development of new enzymes with useful catalytic functions. The evolution of enzymes in biological systems often involves the acquisition of new catalytic or binding properties by an existing protein scaffold. Here we mimic this strategy using the most common fold in enzymes, the α/β-barrel, as the scaffold. By combining an existing binding site for structural elements of phosphoribosylanthranilate with a catalytic template required for isomerase activity, we are able to evolve phosphoribosylanthranilate isomerase activity from the scaffold of indole-3-glycerol-phosphate synthase. We find that targeting the catalytic template for in vitro mutagenesis and recombination, followed by in vivo selection, results in a new phosphoribosylanthranilate isomerase that has catalytic properties similar to those of the natural enzyme, with an even higher specificity constant. Our demonstration of divergent evolution and the widespread occurrence of the α/β-barrel suggest that this scaffold may be a fold of choice for the directed evolution of new biocatalysts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Patthy, L. Protein Evolution (Blackwell Science, Oxford, 1999).
Henikoff, S. et al. Gene families: the taxonomy of protein paralogs and chimeras. Science 278, 609–614 (1997).
Chothia, C. One thousand families for the molecular biologist. Nature 357, 543–544.
Arnold, F. H. & Volkov, A. A. Directed evolution of biocatalysts. Curr. Opin. Chem. Biol. 3, 54– 59 (1999).
Bränden, C. & Tooze, J. Introduction to Protein Structure 2nd edn (Garland, New York, 1999).
Gerlt, J. A. & Babbitt, P. C. Mechanistically diverse enzyme superfamilies: the importance of chemistry in the evolution of catalysis. Curr. Opin. Chem. Biol. 2, 607– 612 (1998).
Holm, L. & Sander, C. An evolutionary treasure: unification of a broad set of amidohydrolases related to urease. Proteins 28, 72–82 (1997).
Hasson, M. S. et al. Evolution of an enzyme active site: the structure of a new crystal form of muconate lactonizing enzyme compared with mandelate racemase and nolase. Proc. Natl Acad. Sci. 95, 10396 –10401 (1998).
Bränden, C. I. The TIM barrel—the most frequently occurring folding motif in proteins. Curr. Opin. Struct. Biol. 1, 978– 983 (1991).
Murzin, A. G., Lesk, A. M. & Chothia, C. Principles determining the structure of beta-sheet barrels in proteins. II. The observed structures. J. Mol. Biol. 236, 1382–1400 (1994).
Murzin, A. G., Lesk, A. M. & Chothia, C. Principles determining the structure of beta-sheet barrels in proteins. I. A theoretical analysis. J. Mol. Biol. 236, 1369–1381 (1994).
Kirschner, K., Szadkowski, H., Jardetzky, T. S. & Hager, V. Phosphoribosylanthranilate isomerase-indoleglycerol-phosphate synthase from Escherichia coli. Methods Enzymol. 142, 386–397 (1987).
Darimont, B., Stehlin, C., Szadkowski, H. & Kirschener, K. Mutational analysis of the active site of indoleglycerol phosphate synthase from Escherichia coli. Protein Sci. 7, 1221–1232 (1998).
Eberhard, M., Tsai-Pflugfelder, M., Bolewska, K., Hommel, U. & Kirschener, K. Indoleglycerol phosphate syntase-phophoribosyl anthranilate isomerase: comparison of the bifunctional enzyme from Escherichia coli with engineered monofunctional domains. Biochemistry 34, 5419–5428 (1995).
Hommel, U., Eberhard, M. & Kirschener, K. Phosphoribosyl anthranilate isomerase catalyzes a reversible Amadori reaction. Biochemistry 34, 5429– 5439 (1995).
Stemmer, W. P. DNA shuffling by random fragmentation and reassembly: in vitro recombination for molecular evolution. Proc. Natl Acad. Sci. USA 91, 12747–10751 (1994).
Stemmer, W. P. Rapid evolution of a protein in vitro by DNA shuffling. Nature 370, 389–391 ( 1994).
Zhao, H. & Arnold, F. H. Optimization of DNA shuffling for high fidelity recombination. Nucleic Acids Res. 25, 1307–1308 (1997).
Shao, Z., Zhao, H., Giver, L. & Arnold, F. H. Random-priming in vitro recombination: an effective tool for directed evolution. Nucleic Acids Res. 26, 681–683 (1998).
Giver, L. & Arnold, F. H. Combinatorial protein design by in vitro recombination. Curr. Opin. Chem. Biol. 2, 335–338 (1998).
Zhao, H., Giver, L., Shao, Z., Affholter, J. A. & Arnold, F. H. Molecular evolution by staggered extension process (StEP) in vitro recombination. Nature Biotechnol. 16, 258–261 (1998).
Crameri, A., Whitehorn, E. A., Tate, E. & Stemmer, W. P. Improved green fluorescent protein by molecular evolution using DNA shuffling. Nature Biotechnol. 14, 315–319 (1996).
Crameri, A., Cwirla, S. & Stemmer, W. P. Construction and evolution of antibody-phage libraries by DNA shuffling. Nature Med. 2, 100– 102 (1996).
Crameri, A., Raillard, S. A., Bermudez, E. & Stemmer, W. P. DNA shuffling of a family of genes from diverse species accelerates directed evolution. Nature 391, 288– 291 (1998).
Tawfik, D. S. & Griffiths, A. D. Man-made cell-like compartments for molecular evolution. Nature Biotechnol. 16, 652–656 (1998).
Kauffman, S. A. (ed.) The Origins of Order (Oxford Univ. Press, New York, 1993).
Wilmanns, M., Priestle, J. P., Niermann, T. & Jansonius, J. N. Three-dimensional structure of the bifunctional enzyme phophoribosylanthranilate isomerase: indoleglycerolphosphate synthase from Escherichia coli refined at 2.0 Å resolution. J. Mol. Biol. 223, 477–507 (1992).
Wilmanns, M., Hyde, C. C., Davies, D. R., Kirschener, K. & Jansonius, J. N. Structural conservation in parallel beta/alpha-barrel enzymes that catalyze three sequential reactions in the pathway of tryptophan biosynthesis. Biochemistry 30, 9161– 9169 (1991).
Knöchel, T. R. et al. The crystal structure of indole-3-glycerol phosphate synthase from the hyperthermophilic archaeon Sulfolobus solfataricus in three different crystal forms: effects of ionic strength. J. Mol. Biol. 262, 502–515 ( 1996).
Luger, K., Hommel, U., Herold, M., Hofsteenge, J. & Kirschner, K. Correct folding of circularly permuted variants of a beta alpha barrel enzyme in vivo. Science 243 , 206–210 (1989).
Stehlin, C., Dahm, A. & Kirschner, K. Deletion mutagenesis as a test of evolutionary relatedness of indoleglycerol phosphate synthase with other TIM barrel enzymes. FEBS Lett. 403, 268–272 (1997).
Neidhart, D. J., Kenyon, G. L., Gerlt, J. A. & Petsko, G. A. Mandelate racemase and muconate lactonizing enzyme are mechanistically distinct and structurally homologous. Nature 347, 692–694 (1990).
Altamirano, M. M., Golbik, R., Zahn, R., Buckle, A. M. & Fersht, A. R. Refolding chromatography with immobilized mini-chaperones. Proc. Natl Acad. Sci. USA 94, 3576– 3578 (1997).
Clarke, L. Isolation of the centromere-linked CDC10 gene by complementation in yeast. Proc. Natl Acad. Sci. USA 77, 2173– 2177 (1980).
Yanofsky, C., Horn, V., Bonner, M. & Stasiowki, S. Polarity and enzyme functions in mutants of the first three genes of the tryptophan operon of Escherichia coli. Genetics 69, 409–433 (1971).
Yanofsky, C. Tryptophan biosynthesis in Escherichia coli. Genetic determination of the proteins involved. J. Am. Med. Assoc. 218, 1026–1035 (1971).
Sterner, R. et al. Phosphoribosyl anthranilate isomerase from Thermotaga maritima is an extremely stable and active homodimer. Protein Sci. 5, 2000–2008 ( 1996).
Winter, G. & Milstein, C. Man-made antibodies. Nature 349, 293–299 ( 1991).
Bisswanger, H., Kirschner, K., Cohn, W., Hager, V. & Hansson, E. N-(5-phosphoribosyl)anthranilate isomerase-indoleglycerol-phosphate synthase. 1. A substrate analogue binds to two different binding sites on the bifunctional enzyme from Escherichia coli. Biochemistry 18, 5946–5953 ( 1979).
Sternberg, N. Display of peptides and proteins on the surface of bacteriophage λ. Proc. Natl. Acad. Sci. USA 92, 1609– 1613 (1995).
Yanofsky, C. & Horn, V. Role of regulatory features of the Trp operon of Escherichia coli in mediating a response to a nutritional shift. J. Bacteriol. 176, 6245– 6254 (1994).
Stephen, A., Gish, W., Miller, W., Myers, E. & Lipman, D. Basic local alignment search tool. J. Mol. Biol. 215 , 403–410 (1990).
Acknowledgements
M.M.A. was a Marie Curie-EU and an EMBO fellow; J.M.B. was a research fellow at Fitzwilliam College and thanks the Royal Society for a University Research Fellowship; C.A. was a Fundación UNAM fellow. We thank P. Barker for valuable advice and discussion, and the Visual Aids Department, especially A. Lenton, for invaluable help.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Altamirano, M., Blackburn, J., Aguayo, C. et al. Directed evolution of new catalytic activity using the α/β-barrel scaffold. Nature 403, 617–622 (2000). https://doi.org/10.1038/35001001
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35001001
This article is cited by
-
Enzyme modification using mutation site prediction method for enhancing the regioselectivity of substrate reaction sites
Scientific Reports (2021)
-
Falsificationism Falsified
Foundations of Science (2006)
-
Correction: retraction: Directed evolution of new catalytic activity using the α/β-barrel scaffold
Nature (2002)
-
The search for the ideal biocatalyst
Nature Biotechnology (2002)
-
Combinatorial and computational challenges for biocatalyst design
Nature (2001)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.