Abstract
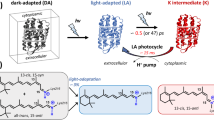
BACTERIORHODOPSIN is found in purple patches on the cell membrane of halophilic bacteria1. Similar in structure to rhodopsin (but differing in function), it consists of a retinal–protein complex. Bacteriorhodopsin is a photosynthetic system which, when it absorbs light, passes through a cycle of several intermediates (Fig. 1) approximately 200 times per second, pumping protons from the inside of the bacterial cell to the outside. The resultant electrochemical gradient drives the phosphorylation of ADP to ATP (ref. 1). The intermediates have been identified by measuring their relatively broad structureless optical absorption spectra2–4 as a function of time after a photolytic flash. The most precise structural information has come from resonance Raman spectra5–8 taken with continuous wave (cw) optical excitation. These studies have provided information about the bR570 and bM412 intermediates only, since they have appreciable steady state concentrations at room temperature under constant illumination. Resonance Raman spectra of these species have also been obtained at liquid N2 temperatures and in ether saturated solutions. These conditions, however, may not be representative of actual physiological conditions. We report here a time-resolved resonance Raman spectrum of an intermediate with a risetime of < 6 ns. Our data show a decrease in the C = C stretching frequency by 10–20 cm−1. From the known inverse correlation between the C = C stretching frequency and the position of the absorption maxima7 for a series of model retinal Schiff bases in various solvents9, the observed C = C Raman band was assigned to the bK590 intermediate, which is known to have a risetime of 10 ps4.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Oesterhelt, D. Angew. Chem. Int. Ed. Engl. B. 15, 17–24 (1976).
Lozier, R. H., Bogolmolni, R. A., & Stoeckenius, W. Biophys. J., 15, 955–962 (1976).
Kung, M. C., De Vault, D., Hess, B. & Oesterhelt, D. Biophys. J. 15, 907–911 (1975).
Kaufmann, K. J., Rentzepis, P. M., Stoeckenius, W. & Lewis, A. Biochem. biophys. Res. Commun. 68, 1109–1115 (1976).
Mendelsohn, R. Nature 243, 22–24 (1973).
Lewis, A., Spoonhower, J., Bogolmolni, R. A., Lozier, R. H. & Stoeckenius, W. Proc. natn. Acad. Sci. U.S.A. 71, 4462–4466 (1974).
Mendelsohn, R., Verma, A. L., Bernstein, H. J. & Kates, M. Can. J. Biochem. 52, 774–781 (1974).
Mendelsohn, R., Biochim. biophys. Acta, 427, 295–301 (1976).
Heyde, M. E., Gill, D., Kilponen, R. G., & Rimai, L. J. Am. Chem. Soc. 93, 6776–6780 (1971).
Oesterhelt, D. & Hess, B. Eur. J. Biochem. 37, 316–326 (1973).
Becher, B. & Cassim, J. Prep. Biochem. 5, 161–178 (1975).
Goldschmidt, C. R., Ottolenghi, M. & Korenstein, R. Biophys. J. 16, 839–843 (1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
CAMPION, A., TERNER, J. & EL-SAYED, M. Time-resolved resonance Raman spectroscopy of bacteriorhodopsin. Nature 265, 659–661 (1977). https://doi.org/10.1038/265659a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/265659a0
This article is cited by
-
Structurally modified bacteriorhodopsin as an efficient bio-sensitizer for solar cell applications
European Biophysics Journal (2019)
-
Flash-induced kinetic infrared spectroscopy applied to biochemical systems
Biophysics of Structure and Mechanism (1980)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.