Abstract
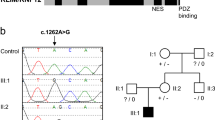
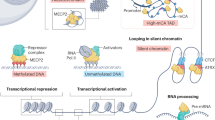
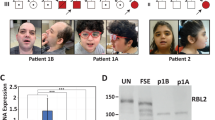
Rett syndrome1 (RTT, MIM 312750) is a progressive neurodevelopmental disorder and one of the most common causes of mental retardation in females, with an incidence of 1 in 10,000–15,000 (ref. 2). Patients with classic RTT appear to develop normally until 6–18 months of age, then gradually lose speech and purposeful hand use, and develop microcephaly, seizures, autism, ataxia, intermittent hyperventilation and stereotypic hand movements3. After initial regression, the condition stabilizes and patients usually survive into adulthood. As RTT occurs almost exclusively in females, it has been proposed that RTT is caused by an X-linked dominant mutation with lethality in hemizygous males3,4,5,6,7,8. Previous exclusion mapping studies using RTT families mapped the locus to Xq28 (refs 6,9,10,11). Using a systematic gene screening approach, we have identified mutations in the gene (MECP2 ) encoding X-linked methyl-CpG-binding protein 2 (MeCP2) as the cause of some cases of RTT. MeCP2 selectively binds CpG dinucleotides in the mammalian genome and mediates transcriptional repression through interaction with histone deacetylase and the corepressor SIN3A (refs 12,13). In 5 of 21 sporadic patients, we found 3 de novo missense mutations in the region encoding the highly conserved methyl-binding domain (MBD) as well as a de novo frameshift and a de novo nonsense mutation, both of which disrupt the transcription repression domain (TRD). In two affected half-sisters of a RTT family, we found segregation of an additional missense mutation not detected in their obligate carrier mother. This suggests that the mother is a germline mosaic for this mutation. Our study reports the first disease-causing mutations in RTT and points to abnormal epigenetic regulation as the mechanism underlying the pathogenesis of RTT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rett, A. Uber ein zerebral-atrophisches Syndrome bei Hyperammonemie (Bruder Hollinek, Vienna, 1966).
Hagberg, B. Rett's syndrome: prevalence and impact on progressive severe mental retardation in girls. Acta Paediatr. Scand. 74, 405– 408 (1985).
Hagberg, B., Aicardi, J., Dias, K. & Ramos, O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: report of 35 cases. Ann. Neurol. 14, 471–479 (1983).
Zoghbi, H. Genetic aspects of Rett syndrome. J. Child Neurol. 3, S76–78 (1988).
Zoghbi, H.Y., Percy, A.K., Schultz, R.J. & Fill, C. Patterns of X chromosome inactivation in the Rett syndrome. Brain Dev. 12, 131–135 (1990).
Ellison, K.A. et al. Examination of X chromosome markers in Rett syndrome: exclusion mapping with a novel variation on multilocus linkage analysis. Am. J. Hum. Genet. 50, 278–287 (1992).
Schanen, N.C. et al. A new Rett syndrome family consistent with X-linked inheritance expands the X chromosome exclusion map. Am. J. Hum. Genet. 61, 634–641 (1997).
Schanen, C. & Francke, U. A severely affected male born into a Rett syndrome kindred supports X-linked inheritance and allows extension of the exclusion map. Am. J. Hum. Genet. 63, 267–269 (1998).
Archidiacono, N. et al. Rett syndrome: exclusion mapping following the hypothesis of germinal mosaicism for new X-linked mutations. Hum. Genet. 86, 604–606 (1991).
Curtis, A.R. et al. X chromosome linkage studies in familial Rett syndrome. Hum. Genet. 90, 551–555 (1993).
Sirianni, N., Naidu, S., Pereira, J., Pillotto, R.F. & Hoffman, E.P. Rett syndrome: confirmation of X-linked dominant inheritance, and localization of the gene to Xq28. Am. J. Hum. Genet. 63, 1552–1558 (1998).
Nan, X. et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393, 386–389 (1998).
Jones, P.L. et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nature Genet. 19, 187– 191 (1998).
Amir, R., Roth Dahle, E., Toniolo, D. & Zoghbi, H.Y. Candidate gene analysis in Rett syndrome and the identification of twenty-one SNPs in Xq. Am. J. Med. Genet. (in press).
Wan, M. & Francke, U. Evaluation of two X chromosomal candidate genes for Rett syndrome: glutamate dehydrogenase-2 (GLUD2) and rab GDP-dissociation inhibitor (GDI1). Am. J. Med. Genet. 78, 169–172 ( 1998).
D'Esposito, M. et al. Isolation, physical mapping, and northern analysis of the X-linked human gene encoding methyl CpG-binding protein, MECP2. Mamm. Genome 7, 533–535 (1996).
Lewis, J.D. et al. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell 69, 905–914 (1992).
Nan, X., Meehan, R.R. & Bird, A. Dissection of the methyl-CpG binding domain from the chromosomal protein MeCP2. Nucleic Acids Res. 21, 4886–4892 (1993).
Nan, X., Campoy, F.J. & Bird, A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell 88, 471 –481 (1997).
Allen, R.C., Zoghbi, H.Y., Moseley, A.B., Rosenblatt, H.M. & Belmont, J.W. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am. J. Hum. Genet. 51, 1229–1239 (1992).
Tate, P., Skarnes, W. & Bird, A. The methyl-CpG binding protein MeCP2 is essential for embryonic development in the mouse. Nature Genet. 12 , 205–208 (1996).
Coy, J.F., Sedlacek, Z., Bachner, D., Delius, H. & Poustka, A. A complex pattern of evolutionary conservation and alternative polyadenylation within the long 3′-untranslated region of the methyl-CpG-binding protein 2 gene (MeCP2) suggests a regulatory role in gene expression. Hum. Mol. Genet. 8, 1253–1262 (1999).
Hendrich, B. & Bird, A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol. Cell. Biol. 18, 6538–6547 ( 1998).
Hendrich, B. et al. Genomic structure and chromosomal mapping of the murine and human mbd1, mbd2, mbd3, and mbd4 genes. Mamm. Genome 10, 906–912 (1999).
Ganguly, A., Rock, M.J. & Prockop, D.J. Conformation-sensitive gel electrophoresis for rapid detection of single-base differences in double-stranded PCR products and DNA fragments: evidence for solvent-induced bends in DNA heteroduplexes. Proc. Natl Acad. Sci. USA 90, 10325– 10329 (1993); erratum: 91, 5217 (1994).
Acknowledgements
We thank the Rett families for their participation and for motivating us as we sought the cause of this disease; the Blue Bird Circle Rett Center, D.G. Glaze and R.J. Schultz for following patients and facilitating sample collection; E.J.R. Dahle for previous research contributions; and A.L. Beaudet for discussions and critical review of the manuscript. This research was supported by the Howard Hughes Medical Institute and NIH grants HD24234 (H.Y.Z. and U.F.) and MRRC HD 24064, the International Rett Syndrome Association (R.A.), the Society for Gynecologic Investigation (I.V.) and the L.M. Chandler Research Fund (M.W.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amir, R., Van den Veyver, I., Wan, M. et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23, 185–188 (1999). https://doi.org/10.1038/13810
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/13810
This article is cited by
-
Psychometric Assessment of the Rett Syndrome Caregiver Assessment of Symptom Severity (RCASS)
Journal of Autism and Developmental Disorders (2024)
-
Nutritional and gastrointestinal manifestations in Rett syndrome: long-term follow-up
European Journal of Pediatrics (2024)
-
Characterization of the Pharmacokinetics and Mass Balance of a Single Oral Dose of Trofinetide in Healthy Male Subjects
Clinical Drug Investigation (2024)
-
qPCR assay optimisation for a clinical study comparing oral health risk in Rett syndrome
European Archives of Paediatric Dentistry (2024)
-
Orthopedic Conditions and Interplay with Functional Abilities and MECP2 Variant Subtype in Rett Syndrome Patients
Journal of Autism and Developmental Disorders (2024)