Abstract
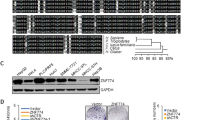
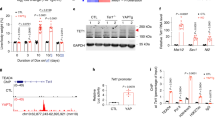
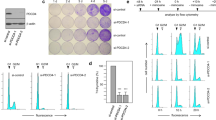
A regulator of the protein phosphatase 2A (PP2A), α4, has been implicated in a variety of functions that regulate many cellular processes. To explore the role of α4 in human cell transformation and tumorigenesis, we show that α4 is highly expressed in human cells transformed by chemical carcinogens including benzo(a)pyrene, aflatoxin B1, N-methyl-N′-nitro-N-nitrosoguanidine, nickel sulfate and in several hepatic and lung cancer cell lines. In addition, overexpression of α4 was detected in 87.5% (74/80) of primary hepatocellular carcinomas, 84.0% (21/25) of primary lung cancers and 81.8% (9/11) of primary breast cancers, indicating that α4 is ubiquitously highly expressed in human cancer. Functional studies revealed that elevated α4 expression results in an increase in cell proliferation, promotion of cell survival and decreased PP2A-attributable activity. Importantly, ectopic expression of α4 permits non-transformed human embryonic kidney cells (HEKTER) and L02R cells to form tumors in immunodeficient mice. Furthermore, we show that the highly expressed α4 in transformed cells or human tumors is not regulated by DNA hypomethylation. A microRNA, miR-34b, that suppresses the expression of α4 through specific binding to the 3′-untranslated region of α4 is downregulated in transformed or human lung tumors. Taken together, these observations identify that α4 possesses an oncogenic function. Reduction of PP2A activity due to an enhanced α4–PP2A interaction contributes directly to chemical carcinogen-induced tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Arroyo JD, Hahn WC . (2005). Involvement of PP2A in viral and cellular transformation. Oncogene 24: 7746–7755.
Bielinski VA, Mumby MC . (2007). Functional analysis of the PP2A subfamily of protein phosphatases in regulating Drosophila S6 kinase. Exp Cell Res 313: 3117–3126.
Bjornsti MA, Houghton PJ . (2004). The TOR pathway: a target for cancer therapy. Nat Rev Cancer 4: 335–348.
Bommer GT, Gerin I, Feng Y, Kaczorowski AJ, Kuick R, Love RE et al. (2007). p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol 17: 1298–1307.
Chen J, Peterson RT, Schreiber SL . (1998). Alpha 4 associates with protein phosphatases 2A, 4, and 6. Biochem Biophys Res Commun 247: 827–832.
Chen W, Arroyo JD, Timmons JC, Possemato R, Hahn WC . (2005). Cancer-associated PP2A Aalpha subunits induce functional haploinsufficiency and tumorigenicity. Cancer Res 65: 8183–8192.
Chen W, Possemato R, Campbell KT, Plattner CA, Pallas DC, Hahn WC . (2004). Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell 5: 127–136.
Colella S, Ohgaki H, Ruediger R, Yang F, Nakamura M, Fujisawa H et al. (2001). Reduced expression of the Aalpha subunit of protein phosphatase 2A in human gliomas in the absence of mutations in the Aalpha and Abeta subunit genes. Int J Cancer 93: 798–804.
Corney DC, Flesken-Nikitin A, Godwin AK, Wang W, Nikitin AY . (2007). MicroRNA-34b and microRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res 67: 8433–8438.
Deichmann M, Polychronidis M, Wacker J, Thome M, Naher H . (2001). The protein phosphatase 2A subunit Bgamma gene is identified to be differentially expressed in malignant melanomas by subtractive suppression hybridization. Melanoma Res 11: 577–585.
Di Como CJ, Arndt KT . (1996). Nutrients, via the Tor proteins, stimulate the association of Tap42 with type 2A phosphatases. Genes Dev 10: 1904–1916.
Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N . (2010). Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta 1804: 433–439.
Eichhorn PJ, Creyghton MP, Bernards R . (2009). Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta 1795: 1–15.
Gibbons JJ, Abraham RT, Yu K . (2009). Mammalian target of rapamycin: discovery of rapamycin reveals a signaling pathway important for normal and cancer cell growth. Semin Oncol 36 (Suppl 3): S3–S17.
Goldberg Y . (1999). Protein phosphatase 2A: who shall regulate the regulator? Biochem Pharmacol 57: 321–328.
Inui S, Sanjo H, Maeda K, Yamamoto H, Miyamoto E, Sakaguchi N . (1998). Ig receptor binding protein 1 (alpha4) is associated with a rapamycin-sensitive signal transduction in lymphocytes through direct binding to the catalytic subunit of protein phosphatase 2A. Blood 92: 539–546.
Irigaray P, Belpomme D . (2010). Basic properties and molecular mechanisms of exogenous chemical carcinogens. Carcinogenesis 31: 135–148.
Janssens V, Goris J . (2001). Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signaling. Biochem J 353: 417–439.
Ji W, Yang L, Yu L, Yuan J, Hu D, Zhang W et al. (2008). Epigenetic silencing of O6-methylguanine DNA methyltransferase gene in NiS-transformed cells. Carcinogenesis 29: 1267–1275.
Kong M, Bui TV, Ditsworth D, Gruber JJ, Goncharov D, Krymskaya VP et al. (2007). The PP2A-associated protein alpha4 plays a critical role in the regulation of cell spreading and migration. J Biol Chem 282: 29712–29720.
Kong M, Ditsworth D, Lindsten T, Thompson CB . (2009). Alpha4 is an essential regulator of PP2A phosphatase activity. Mol Cell 36: 51–60.
Kong M, Fox CJ, Mu J, Solt L, Xu A, Cinalli RM et al. (2004). The PP2A-associated protein alpha4 is an essential inhibitor of apoptosis. Science 306: 695–698.
Liu J, Prickett TD, Elliott E, Meroni G, Brautigan DL . (2001). Phosphorylation and microtubule association of the Opitz syndrome protein mid-1 is regulated by protein phosphatase 2A via binding to the regulatory subunit alpha 4. Proc Natl Acad Sci USA 98: 6650–6655.
Ma RL, Pang YQ, Li WX, Xiao YM, Wei Q, Li DC et al. (2008). Establishment and application of oncogene over expressed human epithelial cell transformation model. Zhonghua Yu Fang Yi Xue Za Zhi 42: 395–399.
McConnell JL, Watkins GR, Soss SE, Franz HS, McCorvey LR, Spiller BW et al. (2010). Alpha4 is a ubiquitin-binding protein that regulates protein serine/threonine phosphatase 2A ubiquitination. Biochemistry 49: 1713–1718.
McDonald WJ, Sangster SM, Moffat LD, Henderson MJ, Too CK . (2010). Alpha4 phosphoprotein interacts with EDD E3 ubiquitin ligase and poly(A)-binding protein. J Cell Biochem 110: 1123–1129.
Meric-Bernstam F, Gonzalez-Angulo AM . (2009). Targeting the mTOR signaling network for cancer therapy. J Clin Oncol 27: 2278–2287.
Mumby M . (2007). PP2A: unveiling a reluctant tumor suppressor. Cell 130: 21–24.
Murata K, Wu J, Brautigan DL . (1997). B cell receptor-associated protein alpha4 displays rapamycin-sensitive binding directly to the catalytic subunit of protein phosphatase 2A. Proc Natl Acad Sci USA 94: 10624–10629.
Onda M, Inui S, Maeda K, Suzuki M, Takahashi E, Sakaguchi N . (1997). Expression and chromosomal localization of the human alpha 4/IGBP1 gene, the structure of which is closely related to the yeast TAP42 protein of the rapamycin-sensitive signal transduction pathway. Genomics 46: 373–378.
Pang Y, Li W, Ma R, Ji W, Wang Q, Li D et al. (2008). Development of human cell models for assessing the carcinogenic potential of chemicals. Toxicol Appl Pharmacol 232: 478–486.
Pigazzi M, Manara E, Baron E, Basso G . (2009). miR-34b targets cyclic AMP-responsive element binding protein in acute myeloid leukemia. Cancer Res 69: 2471–2478.
Prickett TD, Brautigan DL . (2007). Cytokine activation of p38 mitogen-activated protein kinase and apoptosis is opposed by alpha-4 targeting of protein phosphatase 2A for site-specific dephosphorylation of MEK3. Mol Cell Biol 27: 4217–4227.
Sablina AA, Chen W, Arroyo JD, Corral L, Hector M, Bulmer SE et al. (2007). The tumor suppressor PP2A Abeta regulates the RalA GTPase. Cell 129: 969–982.
Shouse GP, Nobumori Y, Liu X . (2010). A B56gamma mutation in lung cancer disrupts the p53-dependent tumor-suppressor function of protein phosphatase 2A. Oncogene 29: 3933–3941.
Smetana JH, Oliveira CL, Jablonka W, Aguiar Pertinhez T, Carneiro FR, Montero-Lomeli M et al. (2006). Low resolution structure of the human alpha4 protein (IgBP1) and studies on the stability of alpha4 and of its yeast ortholog Tap42. Biochim Biophys Acta 1764: 724–734.
Trockenbacher A, Suckow V, Foerster J, Winter J, Krauss S, Ropers HH et al. (2001). MID1, mutated in Opitz syndrome, encodes an ubiquitin ligase that targets phosphatase 2A for degradation. Nat Genet 29: 287–294.
Virshup DM . (2000). Protein phosphatase 2A: a panoply of enzymes. Curr Opin Cell Biol 12: 180–185.
Wang B, Zhao A, Sun L, Zhong X, Zhong J, Wang H et al. (2008). Protein phosphatase PP4 is overexpressed in human breast and lung tumors. Cell Res 18: 974–977.
Westermarck J, Hahn WC . (2008). Multiple pathways regulated by the tumor suppressor PP2A in transformation. Trends Mol Med 14: 152–160.
Wullschleger S, Loewith R, Hall MN . (2006). TOR signaling in growth and metabolism. Cell 124: 471–484.
Yang J, Roe SM, Prickett TD, Brautigan DL, Barford D . (2007). The structure of Tap42/alpha4 reveals a tetratricopeptide repeat-like fold and provides insights into PP2A regulation. Biochemistry 46: 8807–8815.
Yorimitsu T, He C, Wang K, Klionsky DJ . (2009). Tap42-associated protein phosphatase type 2A negatively regulates induction of autophagy. Autophagy 5: 616–624.
Zhang Y, Rohde C, Tierling S, Jurkowski TP, Bock C, Santacruz D et al. (2009). DNA methylation analysis of chromosome 21 gene promoters at single base pair and single allele resolution. PLoS Genet 5: e1000438.
Acknowledgements
We thank Dr Richard Possemato for critical reading of the manuscript. This work was supported by a Distinguished Young Scholar of NSFC (30925029), Key Program of NSFC (30630055) and NSFC (30800930, 30771832, 30901211), National Key Basic Research and Development Program (2010CB912803), National High Technology Research and Development Key Program of China (2008AA062504), Ministry of Health of China (200902006), the Fundamental Research Funds for the Central Universities (10ykjc05 and 10lgzd10) and Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme GDUPS (2010).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, LP., Lai, YD., Li, DC. et al. α4 is highly expressed in carcinogen-transformed human cells and primary human cancers. Oncogene 30, 2943–2953 (2011). https://doi.org/10.1038/onc.2011.20
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.20
Keywords
This article is cited by
-
Protein phosphatase 2A (PP2A): a key phosphatase in the progression of chronic obstructive pulmonary disease (COPD) to lung cancer
Respiratory Research (2019)
-
Loss of protein phosphatase 2A regulatory subunit B56δ promotes spontaneous tumorigenesis in vivo
Oncogene (2018)
-
Expression and regulation of type 2A protein phosphatases and alpha4 signalling in cardiac health and hypertrophy
Basic Research in Cardiology (2017)
-
Predicting targeted drug combinations based on Pareto optimal patterns of coexpression network connectivity
Genome Medicine (2014)
-
Structural basis of protein phosphatase 2A stable latency
Nature Communications (2013)