Key Points
-
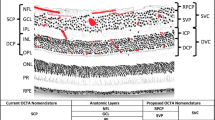
The macula is the central retinal region specialized for high acuity vision.
-
The retina has the highest oxygen consumption of any tissue in the body and together with the underlying retinal pigment epithelium (RPE) lives a metabolically precarious existence.
-
The retina is the only region of the human nervous system that can be observed directly. Two new, non-invasive imaging technologies, optical coherence tomography (OCT) and confocal scanning laser ophthalmoscopy (cSLO), allow the clinician to view the retina with nearly cellular resolution.
-
Genes for all of the main early-onset types of macular degeneration have been identified, and they reveal a role for the following structures or processes in macular disease: anion flux in the RPE; retinoid cycling and the production of a phototoxic retinoid-derived side product (A2E); the sub-RPE extracellular matrix; pro-angiogenic signalling; and long-chain fatty acid synthesis.
-
Animal models implicate oxidative, and in particular photo-oxidative, damage and immune system defects in the pathogenesis of macular degeneration.
-
Sub-RPE deposits are associated with age-related macular degeneration (AMD). The protein composition of these deposits and the results of experiments using knockout mice implicate the immune response in the pathogenesis of AMD.
-
Three loci with large effects on AMD risk have been identified. Two of these loci code for regulators of the complement cascade (complement factors B and H), directly implicating misregulation of the complement cascade in AMD.
-
Current therapies for AMD focus on blocking the growth of new blood vessels from the choroid into the retina (choroidal neovascularization). A major challenge for the future will be to develop therapies that target earlier stages in the progression of macular disease.
Abstract
The central retina mediates high acuity vision, and its progressive dysfunction due to macular degeneration is the leading cause of visual disability among adults in industrialized societies. Here, we summarize recent progress in understanding the pathophysiology of macular degeneration and the implications of this new knowledge for treatment and prevention. The past decade has witnessed remarkable advances in this field, including the development of new, non-invasive retinal imaging technologies, the development of animal models for macular disease, and the isolation of many of the genes responsible for both early- and late-onset macular diseases. These advances have set the stage for the development of effective mechanism-based therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Warburg, O. Uber die klassifizierung tierischer gewebe nach ihrem stoffwechsel. Biochem. Z. 184, 484–488 (1928) (in German).
Linsenmeier, R. A. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J. Gen. Physiol. 88, 521–542 (1986).
Marmor, M. F. & Wolfensberger, T. J. The Retinal Pigment Epithelium (Oxford Univ. Press, Oxford, 1998). The classic reference work for RPE biology.
Marquardt, A. et al. Mutations in a novel gene, VMD2, encoding a protein of unknown properties cause juvenile-onset vitelliform macular dystrophy (Best's disease). Hum. Mol. Genet. 7, 1517–1525 (1998).
Petrukhin, K. et al. Identification of the gene responsible for Best macular dystrophy. Nature Genet. 19, 241–247 (1998).
Sun, H., Tsunenari, T., Yau, K. W. & Nathans, J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc. Natl Acad. Sci. USA 99, 4008–4013 (2002).
Marmorstein, A. D. et al. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc. Natl Acad. Sci. USA 97, 12758–12763 (2000).
Weber, B. H., Vogt, G., Pruett, R. C., Stohr, H. & Felbor, U. Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby's fundus dystrophy. Nature Genet. 8, 352–356 (1994).
Jacobson, S. G. et al. Night blindness in Sorsby's fundus dystrophy reversed by vitamin A. Nature Genet. 11, 27–32 (1995).
Stone, E. M. et al. A single EFEMP1 mutation associated with both Malattia Leventinese and Doyne honeycomb retinal dystrophy. Nature Genet. 22, 199–202 (1999).
Klenotic, P. A., Munier, F. L., Marmorstein, L. Y. & Anand-Apte, B. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is a binding partner of epithelial growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1). Implications for macular degenerations. J. Biol. Chem. 279, 30469–30473 (2004).
Qi, J. H. et al. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nature Med. 9, 407–415 (2003).
Judge, D. P. & Dietz, H. C. Marfan's syndrome. Lancet 366, 1965–1976 (2005).
Allikmets, R. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nature Genet. 17, 236–246 (1997).
Illing, M., Molday, L. L. & Molday, R. S. The 220-kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J. Biol. Chem. 272, 10303–10310 (1997).
Sun, H. & Nathans, J. Stargardt's ABCR is localized to the disc membrane of retinal rod outer segments. Nature Genet. 17, 15–16 (1997).
Molday, L. L., Rabin, A. R. & Molday, R. S. ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nature Genet. 25, 257–258 (2000).
Sun, H., Molday, R. S. & Nathans, J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J. Biol. Chem. 274, 8269–8281 (1999). One of the initial descriptions of ABCR's role in the retinoid cycle and in the pathophysiology of Stargardt disease, based on experiments with purified and reconstituted ABCR in vitro.
Weng, J. et al. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell 98, 13–23 (1999). One of the initial descriptions of ABCR's role in the retinoid cycle and in the pathophysiology of Stargardt disease, based on experiments with Abcr−/− mice.
Fishman, G. A., Farbman, J. S. & Alexander, K. R. Delayed rod dark adaptation in patients with Stargardt's disease. Ophthalmology 98, 957–962 (1991).
Delori, F. C. et al. In vivo measurement of lipofuscin in Stargardt's disease — Fundus flavimaculatus. Invest. Ophthalmol. Vis. Sci. 36, 2327–2331 (1995).
Mata, N. L., Weng, J. & Travis, G. H. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc. Natl Acad. Sci. USA 97, 7154–7159 (2000). Demonstrates the accumulation of A2E in both a human Stargardt disease patient's eyes and in a mouse model of Stargardt disease.
Mata, N. L. et al. Delayed dark-adaptation and lipofuscin accumulation in abcr+/− mice: implications for involvement of ABCR in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 42, 1685–1690 (2001).
Parish, C. A., Hashimoto, M., Nakanishi, K., Dillon, J. & Sparrow, J. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc. Natl Acad. Sci. USA 95, 14609–14613 (1998).
Sparrow, J. R. et al. A2E, a byproduct of the visual cycle. Vision. Res. 43, 2983–2990 (2003).
Sparrow, J. R. et al. Involvement of oxidative mechanisms in blue-light-induced damage to A2E-laden RPE. Invest. Ophthalmol. Vis. Sci. 43, 1222–1227 (2002). Demonstration of the cellular phototoxicity of A2E.
Radu, R. A., Mata, N. L., Bagla, A. & Travis, G. H. Light exposure stimulates formation of A2E oxiranes in a mouse model of Stargardt's macular degeneration. Proc. Natl Acad. Sci. USA 101, 5928–5933 (2004).
Edwards, A. O., Donoso, L. A. & Ritter, R. A novel gene for autosomal dominant Stargardt-like macular dystrophy with homology to the SUR4 protein family. Invest. Ophthalmol. Vis. Sci. 42, 2652–2663 (2001).
Zhang, K. et al. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nature Genet. 27, 89–93 (2001).
Klein, R. in Age-Related Macular Degeneration (eds Berger, J. W., Fine, S. L. & Maguire, M. G.) 31–56 (Mosby, St. Louis, 1999). A highly readable summary of AMD epidemiology.
Maguire, M. G. in Age-Related Macular Degeneration (eds Berger, J. W., Fine, S. L. & Maguire, M. G.) 17–30 (Mosby, St. Louis, 1999).
Eisner, A., Fleming, S. A., Klein, M. L. & Mauldin, W. M. Sensitivities in older eyes with good acuity: eyes whose fellow eye has exudative AMD. Invest. Ophthalmol. Vis. Sci. 28, 1832–1837 (1987).
Eisner, A., Stoumbos, V. D., Klein, M. L. & Fleming, S. A. Relations between fundus appearance and function. Eyes whose fellow eye has exudative age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 32, 8–20 (1991).
Klein, R., Klein, B. E. & Linton, K. L. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 99, 933–943 (1992).
Mitchell, P., Smith, W., Attebo, K. & Wang, J. J. Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology 102, 1450–1460 (1995).
Vingerling, J. R. et al. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology 102, 205–210 (1995).
Seddon, J. M., Ajani, U. A. & Mitchell, B. D. Familial aggregation of age-related maculopathy. Am. J. Ophthalmol. 123, 199–206 (1997).
Thornton, J. et al. Smoking and age-related macular degeneration: a review of association. Eye 19, 935–944 (2005).
Heiba, I. M., Elston, R. C., Klein, B. E. & Klein, R. Sibling correlations and segregation analysis of age-related maculopathy: the Beaver Dam Eye Study. Genet. Epidemiol. 11, 51–67 (1994).
Meyers, S. M., Greene, T. & Gutman, F. A. A twin study of age-related macular degeneration. Am. J. Ophthalmol. 120, 757–766 (1995).
Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, β-carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 119, 1417–1436 (2001).
Guymer, R. & Bird, A. C. in The Retinal Pigment Epithelium (eds Marmor, M. F. & Wolfensberger, T. J.) 693–705 (Oxford Univ. Press, Oxford, 1998).
Marshall, J., Hussain, A. A., Starita, C., Moore, D. J. & Patmore, A. L. in The Retinal Pigment Epithelium (eds Marmor, M. F. & Wolfensberger, T. J.) 669–692 (Oxford Univ. Press, Oxford, 1998)
Zarbin, M. A. Current concepts in the pathogenesis of age-related macular degeneration. Arch. Ophthalmol. 122, 598–614 (2004).
Anderson, D. H., Mullins, R. F., Hageman, G. S. & Johnson, L. V. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 134, 411–431 (2002).
Mullins, R. F., Russell, S. R., Anderson, D. H. & Hageman, G. S. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 14, 835–846 (2000). One of the first descriptions of inflammation-associated protein deposits in drusen.
Hageman, G. S. et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE–Bruch's membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 20, 705–732 (2001).
Johnson, L. V., Leitner, W. P., Staples, M. K. & Anderson, D. H. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp. Eye Res. 73, 887–896 (2001). Along with reference 46, this paper was one of the first descriptions of inflammation-associated protein deposits in drusen.
Johnson, L. V. et al. The Alzheimer's Aβ peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc. Natl Acad. Sci. USA 99, 11830–11835 (2002).
Crabb, J. W. et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc. Natl Acad. Sci. USA 99, 14682–14687 (2002). Describes the use of mass spectrometry to define the composition of microdissected drusen, revealing a wide variety of components.
Nozaki, M. et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl Acad. Sci. USA 103, 2328–2333 (2006).
Bressler, N. M., Bressler, S. B., West, S. K., Fine, S. L. & Taylor, H. R. The grading and prevalence of macular degeneration in Chesapeake Bay watermen. Arch. Ophthalmol. 107, 847–852 (1989).
Klein, R., Klein, B. E., Jensen, S. C. & Meuer, S. M. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology 104, 7–21 (1997).
Penfold, P. L., Killingsworth, M. C. & Sarks, S. H. Senile macular degeneration: the involvement of immunocompetent cells. Graefes Arch. Clin. Exp. Ophthalmol. 223, 69–76 (1985).
Penfold, P. L., Madigan, M. C., Gillies, M. C. & Provis, J. M. Immunological and aetiological aspects of macular degeneration. Prog. Retin. Eye Res. 20, 385–414 (2001).
Duvall-Young, J., MacDonald, M. K. & McKechnie, N. M. Fundus changes in (type II) mesangiocapillary glomerulonephritis simulating drusen: a histopathological report. Br. J. Ophthalmol. 73, 297–302 (1989).
Duvall-Young, J., Short, C. D., Raines, M. F., Gokal, R. & Lawler, W. Fundus changes in mesangiocapillary glomerulonephritis type II: clinical and fluorescein angiographic findings. Br. J. Ophthalmol. 73, 900–906 (1989).
Leys, A. et al. Subretinal neovascular membranes associated with chronic membranoproliferative glomerulonephritis type II. Graefes Arch. Clin. Exp. Ophthalmol. 228, 499–504 (1990).
Leys, A. et al. Fundus changes in membranoproliferative glomerulonephritis type II. A fluorescein angiographic study of 23 patients. Graefes Arch. Clin. Exp. Ophthalmol. 229, 406–410 (1991).
Mullins, R. F., Aptsiauri, N. & Hageman, G. S. Structure and composition of drusen associated with glomerulonephritis: implications for the role of complement activation in drusen biogenesis. Eye 15, 390–395 (2001).
Zipfel, P. F., Heinen, S., Jozsi, M. & Skerka, C. Complement and diseases: defective alternative pathway control results in kidney and eye diseases. Mol. Immunol. 43, 97–106 (2006).
Roitt, I. M. Essential Immunology (Blackwell Scientific, Oxford, 1994).
Holers, V. M. in Clinical Immunology: Principles and Practice (eds Rich, R. R., et al.) 363–391 (Mosby, St. Louis, 1996).
Dragon-Durey, M. A. et al. Heterozygous and homozygous factor H deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J. Am. Soc. Nephrol. 15, 787–795 (2004).
Delori, F. C., Goger, D. G. & Dorey, C. K. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest. Ophthalmol. Vis. Sci. 42, 1855–1866 (2001).
von Ruckmann, A., Fitzke, F. W. & Bird, A. C. Fundus autofluorescence in age-related macular disease imaged with a laser scanning ophthalmoscope. Invest. Ophthalmol. Vis. Sci. 38, 478–486 (1997).
Hopkins, J., Walsh, A. & Chakravarthy, U. Fundus autofluorescence in age-related macular degeneration: an epiphenomenon? Invest. Ophthalmol. Vis. Sci. 47, 2269–2271 (2006).
Allikmets, R. Molecular genetics of age-related macular degeneration: current status. Eur. J. Ophthalmol. 9, 255–265 (1999).
Allikmets, R. Further evidence for an association of ABCR alleles with age-related macular degeneration. The International ABCR Screening Consortium. Am. J. Hum. Genet. 67, 487–491 (2000).
Shroyer, N. F., Lewis, R. A., Yatsenko, A. N., Wensel, T. G. & Lupski, J. R. Cosegregation and functional analysis of mutant ABCR (ABCA4) alleles in families that manifest both Stargardt disease and age-related macular degeneration. Hum. Mol. Genet. 10, 2671–2678 (2001).
Stone, E. M. et al. Missense variations in the fibulin 5 gene and age-related macular degeneration. N. Engl. J. Med. 351, 346–353 (2004).
Fisher, S. A. et al. Meta-analysis of genome scans of age-related macular degeneration. Hum. Mol. Genet. 14, 2257–2264 (2005). Combines the data from multiple whole genome linkage scans to reveal a set of promising candidate regions for AMD susceptibility genes.
Edwards, A. O. et al. Complement factor H polymorphism and age-related macular degeneration. Science 308, 421–424 (2005).
Hageman, G. S. et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl Acad. Sci. USA 102, 7227–7232 (2005).
Haines, J. L. et al. Complement factor H variant increases the risk of age-related macular degeneration. Science 308, 419–421 (2005).
Klein, R. J. et al. Complement factor H polymorphism in age-related macular degeneration. Science 308, 385–389 (2005). References 73–76 are four initial reports of linkage between AMD susceptibility and sequence variation in the CFH gene.
Herbert, A. P., Uhrin, D., Lyon, M., Pangburn, M. K. & Barlow, P. N. Disease-associated sequence variations congregate in a polyanion recognition patch on human factor H revealed in three-dimensional structure. J. Biol. Chem. 281, 16512–16520 (2006).
Saunders, R. E., Goodship, T. H., Zipfel, P. F. & Perkins, S. J. An interactive web database of factor H-associated hemolytic uremic syndrome mutations: insights into the structural consequences of disease-associated mutations. Hum. Mutat. 27, 21–30 (2006).
Li, M. et al. CFH haplotypes without the Y402H coding variant show strong association with susceptibility to age-related macular degeneration. Nature Genet. 38, 1049–1054 (2006).
Jakobsdottir, J. et al. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am. J. Hum. Genet. 77, 389–407 (2005).
Rivera, A. et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum. Mol. Genet. 14, 3227–3236 (2005).
Schmidt, S. et al. Cigarette smoking strongly modifies the association of LOC387715 and age-related macular degeneration. Am. J. Hum. Genet. 78, 852–864 (2006). Along with reference 81, this work describes a strong association between a second locus, unlinked to CFH , and AMD.
Gold, B. et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nature Genet. 38, 458–462 (2006). The first description of AMD susceptibility associated with BF gene variation.
Ishibashi, T., Miller, H., Orr, G., Sorgente, N. & Ryan, S. J. Morphologic observations on experimental subretinal neovascularization in the monkey. Invest. Ophthalmol. Vis. Sci. 28, 1116–1130 (1987).
Blaauwgeers, H. G. et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am. J. Pathol. 155, 421–428 (1999).
Dawson, D. W. et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science 285, 245–248 (1999).
Oshima, Y. et al. Increased expression of VEGF in retinal pigmented epithelial cells is not sufficient to cause choroidal neovascularization. J. Cell Physiol. 201, 393–400 (2004).
Bora, P. S. et al. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J. Immunol. 174, 491–497 (2005). A convincing demonstration that there is an immunological component to experimental choroidal neovascularization, even if it is induced by a laser burn.
Espinosa-Heidmann, D. G., Sall, J., Hernandez, E. P. & Cousins, S. W. Basal laminar deposit formation in APO B100 transgenic mice: complex interactions between dietary fat, blue light, and vitamin E. Invest. Ophthalmol. Vis. Sci. 45, 260–266 (2004).
Espinosa-Heidmann, D. G. et al. Cigarette smoke-related oxidants and the development of sub-RPE deposits in an experimental animal model of dry AMD. Invest. Ophthalmol. Vis. Sci. 47, 729–737 (2006).
Hahn, P. et al. Disruption of ceruloplasmin and hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration. Proc. Natl Acad. Sci. USA 101, 13850–13855 (2004).
Malek, G. et al. Apolipoprotein E allele-dependent pathogenesis: a model for age-related retinal degeneration. Proc. Natl Acad. Sci. USA 102, 11900–11905 (2005).
Imamura, Y. et al. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proc. Natl Acad. Sci. USA 103, 11282–11287 (2006). Describes genetic evidence that oxidative and photo-oxidative damage induce drusen formation in a mouse model of AMD.
Ambati, J. et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nature Med. 9, 1390–1397 (2003).
Jansen, J. H., Hogasen, K. & Grondahl, A. M. Porcine membranoproliferative glomerulonephritis type II: an autosomal recessive deficiency of factor H. Vet. Rec. 137, 240–244 (1995).
Pickering, M. C. et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nature Genet. 31, 424–428 (2002).
Weber, B. H. et al. A mouse model for Sorsby fundus dystrophy. Invest. Ophthalmol. Vis. Sci. 43, 2732–2740 (2002).
Karan, G. et al. Lipofuscin accumulation, abnormal electrophysiology, and photoreceptor degeneration in mutant ELOVL4 transgenic mice: a model for macular degeneration. Proc. Natl Acad. Sci. USA 102, 4164–4169 (2005).
Radu, R. A. et al. Treatment with isotretinoin inhibits lipofuscin accumulation in a mouse model of recessive Stargardt's macular degeneration. Proc. Natl Acad. Sci. USA 100, 4742–4747 (2003). Presents a mouse model of Stargardt disease in which pharmacological inhibition of RPE65 blocks A2E accumulation.
Maiti, P. et al. Small molecule RPE65 antagonists limit the visual cycle and prevent lipofuscin formation. Biochemistry 45, 852–860 (2006).
Umeda, S. et al. Molecular composition of drusen and possible involvement of anti-retinal autoimmunity in two different forms of macular degeneration in cynomolgus monkey (Macaca fascicularis). FASEB J. 19, 1683–1685 (2005).
Gass, J. D. Photocoagulation of macular lesions. Trans. Am. Acad. Ophthalmol. Otolaryngol. 75, 580–608 (1971).
Berger, J. W. & Fine, S. L. in Age-Related Macular Degeneration (eds Berger, J. W., Fine, S. L. & Maguire, M. G.) 31–56 (Mosby, St. Louis, 1999).
Michels, S. et al. Comparison of early retreatment with the standard regimen in verteporfin therapy of neovascular age-related macular degeneration. Ophthalmology 112, 2070–2075 (2005).
Eyetech Study Group. Anti-vascular endothelial growth factor therapy for subfoveal choroidal neovascularization secondary to age-related macular degeneration: phase II study results. Ophthalmology 110, 979–986 (2003).
Avery, R. L. et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 113, 363–372 (2006).
Rosenfeld, P. J., Heier, J. S., Hantsbarger, G. & Shams, N. Tolerability and efficacy of multiple escalating doses of ranibizumab (Lucentis) for neovascular age-related macular degeneration. Ophthalmology 113, 632 (2006).
Ferrara, N. Vascular endothelial growth factor: basic science and clinical progress. Endocr. Rev. 25, 581–611 (2004).
Gass, J. D. Drusen and disciform macular detachment and degeneration. Arch. Ophthalmol. 90, 206–217 (1973).
Cleasby, G. W., Nakanishi, A. S. & Norris, J. L. Prophylactic photocoagulation of the fellow eye in exudative senile maculopathy. A preliminary report. Mod. Probl. Ophthalmol. 20, 141–147 (1979).
Wetzig, P. C. Treatment of drusen-related aging macular degeneration by photocoagulation. Trans. Am. Ophthalmol. Soc. 86, 276–290 (1988).
Wetzig, P. C. Photocoagulation of drusen-related macular degeneration: a long-term outcome. Trans. Am. Ophthalmol. Soc. 92, 299–303 (1994).
Duvall, J. & Tso, M. O. Cellular mechanisms of resolution of drusen after laser coagulation. An experimental study. Arch. Ophthalmol. 103, 694–703 (1985).
Hsu, J., Maguire, M. G. & Fine, S. L. Laser prophylaxis for age-related macular degeneration. Can. J. Ophthalmol. 40, 320–331 (2005).
Monsonego, A. & Weiner, H. L. Immunotherapeutic approaches to Alzheimer's disease. Science 302, 834–838 (2003).
Wilcock, D. M. et al. Passive amyloid immunotherapy clears amyloid and transiently activates microglia in a transgenic mouse model of amyloid deposition. J. Neurosci. 24, 6144–6151 (2004).
Ornish, D. et al. Intensive lifestyle changes for reversal of coronary heart disease. J. Am. Med. Assoc. 280, 2001–2007 (1998).
Nissen, S. E. et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N. Engl. J. Med. 352, 29–38 (2005).
Wong, J., Patel, R. A. & Kowey, P. R. The clinical use of angiotensin-converting enzyme inhibitors. Prog. Cardiovasc. Dis. 47, 116–130 (2004).
Spaltenstein, A., Kazmierski, W. M., Miller, J. F. & Samano, V. Discovery of next generation inhibitors of HIV protease. Curr. Top. Med. Chem. 5, 1589–1607 (2005).
Radu, R. A. et al. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest. Ophthalmol. Vis. Sci. 46, 4393–4401 (2005).
de Kruif, M. D., van Gorp, E. C., Keller, T. T., Ossewaarde, J. M. & ten Cate, H. Chlamydia pneumoniae infections in mouse models: relevance for atherosclerosis research. Cardiovasc. Res. 65, 317–327 (2005).
Campbell, L. A. & Kuo, C. C. Chlamydia pneumoniae — an infectious risk factor for atherosclerosis? Nature Rev. Microbiol. 2, 23–32 (2004).
Kalayoglu, M. V., Galvan, C., Mahdi, O. S., Byrne, G. I. & Mansour, S. Serological association between Chlamydia pneumoniae infection and age-related macular degeneration. Arch. Ophthalmol. 121, 478–482 (2003).
Kalayoglu, M. V. et al. Identification of Chlamydia pneumoniae within human choroidal neovascular membranes secondary to age-related macular degeneration. Graefes. Arch. Clin. Exp. Ophthalmol. 243, 1080–1090 (2005).
Pieramici, D. J. & Bressler, S. B. in Age-Related Macular Degeneration (eds Berger, J. W., Fine, S. L. & Maguire, M. G.) 219–236 (Mosby, St. Louis, 1999).
Yannuzzi, L. A. et al. Ophthalmic fundus imaging: today and beyond. Am. J. Ophthalmol. 137, 511–524 (2004).
Sharp, P. F., Manivannan, A., Xu, H. & Forrester, J. V. The scanning laser ophthalmoscope — a review of its role in bioscience and medicine. Phys. Med. Biol. 49, 1085–1096 (2004).
Thomas, D. & Duguid, G. Optical coherence tomography — a review of the principles and contemporary uses in retinal investigation. Eye 18, 561–570 (2004).
Webb, R. H., Hughes, G. W. & Delori, F. C. Confocal scanning laser ophthalmoscope. Appl. Opt. 26, 1492–1499 (1987). One of the original descriptions of the cSLO technology.
Bindewald, A. et al. Classification of fundus autofluorescence patterns in early age-related macular disease. Invest. Ophthalmol. Vis. Sci. 46, 3309–3314 (2005).
Zhang, Y. & Roorda, A. Evaluating the lateral resolution of the adaptive optics scanning laser ophthalmoscope. J. Biomed. Opt. 11, 14002 (2006).
Hossain, P. et al. In vivo cell tracking by scanning laser ophthalmoscopy: quantification of leukocyte kinetics. Invest. Ophthalmol. Vis. Sci. 39, 1879–1887 (1998).
Martin, J. A. & Roorda, A. Direct and noninvasive assessment of parafoveal capillary leukocyte velocity. Ophthalmology 112, 2219–2224 (2005).
Hammer, D. X. et al. Compact scanning laser ophthalmoscope with high-speed retinal tracker. Appl. Opt. 42, 4621–4632 (2003).
Huang, D. et al. Optical coherence tomography. Science 254, 1178–1181 (1991). The invention of OCT.
Schmidt-Erfurth, U. et al. Three-dimensional ultrahigh-resolution optical coherence tomography of macular diseases. Invest. Ophthalmol. Vis. Sci. 46, 3393–3402 (2005).
Cucu, R. G., Podoleanu, A. G., Rogers, J. A., Pedro, J. & Rosen, R. B. Combined confocal/en face T-scan-based ultrahigh-resolution optical coherence tomography in vivo retinal imaging. Opt. Lett. 31, 1684–1686 (2006).
Fernandez, E. J. et al. Three-dimensional adaptive optics ultrahigh-resolution optical coherence tomography using a liquid crystal spatial light modulator. Vision. Res. 45, 3432–3444 (2005). Along with reference 138, this paper provides a recent description of state-of-the-art ultra-high- resolution OCT.
Podoleanu, A. G. et al. Combined multiplanar optical coherence tomography and confocal scanning ophthalmoscopy. J. Biomed. Opt. 9, 86–93 (2004).
Osterberg, G. Topography of the layer of rods and cones in the human retina. Acta Ophthalmol. Suppl. 6, 1–101 (1935).
Sparrow, J. R., Nakanishi, K. & Parish, C. A. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest. Ophthalmol. Vis. Sci. 41, 1981–1989 (2000).
Acknowledgements
The authors thank the following colleagues for permission to reproduce images: R. Cucu (Box 1c); G. Hageman (Fig. 5a–c); F. Holz (Box 1b); L. Johnson (Fig. 5d); R. Lewis (Fig. 2a,c); J. Sparrow (Fig. 3b); E. Stone (Fig. 2b; Box 1a); and H. Sun (Fig. 4a). The authors are supported by the National Institutes of Health (National Eye Institute) and the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Jeremy Nathans is a paid consultant for the Ophthalmology Group at the Novartis Research Institute.
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Glossary
- Metalloproteinase
-
A proteinase that has a metal ion at its active site.
- Druse/drusen
-
Discrete sub-retinal pigment epithelium deposits composed of a complex mixture of lipid and protein. From German, meaning 'rock' or 'geode'.
- Fundus
-
The posterior region of the eye, including the retina, retinal pigment epithelium and choroid.
- Hypercholesterolemia
-
Elevated serum cholesterol levels.
- Confocal scanning laser ophthalmoscopy
-
(cSLO). A non-invasive technique for fundus examination that produces en face images of the retina at high spatial resolution.
- Logarithm of the odds (LOD) score
-
The standard statistical test for genetic linkage, whereby the likelihood of bone fide linkage is compared to the probability that the data reflect a chance association.
- Single nucleotide polymorphism
-
(SNP). One of the most common genetic variations in humans. It occurs when a single nucleotide (for example, thymine) replaces one of the other three nucleotides (adenine, guanine or cytosine).
- Haplotype
-
Clustered DNA sequence variants that were present together on a common ancestral chromosome. Because of their close proximity they are generally inherited together and serve to define the ancestral chromosomal region.
- Major histocompatibility complex
-
(MHC). There are two classes of MHC molecules. MHC class I molecules are found on the surface of most cells and present proteins that are generated in the cytosol to T lymphocytes. MHC class II molecules are expressed only on the surface of activated antigen-presenting cells, and they present peptides that have been degraded in cellular vesicles to T cells.
- Hyperlipidemia
-
Elevated serum lipid levels.
- Optical coherence tomography
-
(OCT). A non-invasive technique for fundus examination that produces cross-sectional images of the retina, retinal pigment epithelium and choroid.
Rights and permissions
About this article
Cite this article
Rattner, A., Nathans, J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci 7, 860–872 (2006). https://doi.org/10.1038/nrn2007
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn2007
This article is cited by
-
Association of HTRA1 and CFH gene polymorphisms with age-related macular degeneration in Ningbo, China
International Ophthalmology (2021)
-
Light microscopic evidence of in vivo differentiation from the transplanted inferior turbinate-derived stem cell into the rod photoreceptor in degenerating retina of the mouse
Applied Microscopy (2020)
-
TGF-β participates choroid neovascularization through Smad2/3-VEGF/TNF-α signaling in mice with Laser-induced wet age-related macular degeneration
Scientific Reports (2017)
-
Functional and morphological evaluation of blue light-emitting diode-induced retinal degeneration in mice
Graefe's Archive for Clinical and Experimental Ophthalmology (2016)
-
Generation of retinal ganglion cells with functional axons from human induced pluripotent stem cells
Scientific Reports (2015)