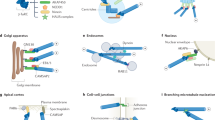
Abstract
Microtubules have long been implicated in the polarization of migrating cells, but how they carry out this role is unclear. Here, we propose that microtubules determine cell polarity by modulating the pattern of adhesions that a cell develops with the underlying matrix, through focal inhibitions of contractility.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Vasiliev, J. M. et al. Effect of colcemid on the locomotory behaviour of fibroblasts. J. Embryol. Exp. Morphol. 24, 625–640 (1970).
Small, J. V., Stradal, T., Vignal, E. & Rottner, K. The lamellipodium: where motility begins. Trends Cell Biol. 12, 112–120 (2002).
Chen, W. -T. Mechanism of retraction of the trailing edge during fibroblast movement. J. Cell Biol. 90, 187–200 (1981).
Jay, P. Y., Pham, P. A., Wong, S. A. & Elson, E. L. A mechanical function of myosin II in cell motility. J. Cell Sci. 108, 387–393 (1995).
Geiger, B., Bershadsky, A., Pankov, R. & Yamada, K. M. Transmembrane extracellular matrix–cytoskeleton crosstalk. Nature Rev. Mol. Cell Biol. 2, 793–805 (2001).
Nobes, C. D. & Hall, A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62 (1995).
Rottner, K., Hall, A. & Small, J. V. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol. 9, 640–648 (1999).
Smilenov, L. B., Mikhailov, A., Pelham, R. J., Marcantonio, E. E. & Gundersen, G. G. Focal adhesion motility revealed in stationary fibroblasts. Science 286, 1172–1174 (1999).
Zamir, E. et al. Dynamics and segregation of cell–matrix adhesions in cultured fibroblasts. Nature Cell Biol. 2, 191–196 (2000).
Ballestrem, C., Hinz, B., Imhof, B. A. & Wehrle-Haller, B. Marching at the front and dragging behind: differential αVβ3-integrin turnover regulates focal adhesion behavior. J. Cell Biol. 155, 1319–1332 (2001).
Kaverina, I. et al. Enforced polarisation and locomotion of fibroblasts lacking microtubules. Curr. Biol. 10, 739–742 (2000).
Anderson, K. I. & Cross, R. Contact dynamics during keratocyte motility. Curr. Biol. 10, 253–260 (2000).
Chrzanowska-Wodnicka, M. & Burridge, K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 133, 1403–1415 (1996).
Geiger, B. & Bershadsky, A. Assembly and mechanosensory function of focal contacts. Curr. Opin. Cell Biol. 13, 584–592 (2001).
Ridley, A. J. & Hall, A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70, 389–399 (1992).
Helfman, D. M. et al. Caldesmon inhibits nonmuscle cell contractility and interferes with the formation of focal adhesions. Mol. Biol. Cell 10, 3097–3112 (1999).
Webb, D. J., Parsons, J. T. & Horwitz, A. F. Adhesion assembly, disassembly and turnover in migrating cells — over and over and over again. Nature Cell Biol. 4, E97–E100 (2002).
Riveline, D. et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 153, 1175–1186 (2001).
Kaverina, I. et. al. Tensile stress stimulates microtubule outgrowth in living cells. J. Cell Sci. 115, 2283–2291 (2002).
Watanabe, N., Kato, T., Fujita, A., Ishizaki, T. & Narumiya, S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nature Cell Biol. 1, 136–143 (1999).
Beningo, K. A., Dembo, M., Kaverina, I., Small, J. V. & Wang, Y. L. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol. 153, 881–888 (2001).
Balaban, N. Q. et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nature Cell Biol. 3, 466–472 (2001).
Beningo, K. A. & Wang, Y. L. Flexible substrata for the detection of cellular traction forces. Trends Cell Biol. 12, 79–84 (2002).
Oliver, T., Dembo, M. & Jacobson, K. Separation of propulsive and adhesive traction stresses in locomoting keratocytes. J. Cell Biol. 145, 589–604 (1999).
Bershadsky, A., Chausovsky, A., Becker, E., Lyubimova, A. & Geiger, B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr. Biol. 6, 1279–1289 (1996).
Enomoto, T. Microtubule disruption induces the formation of actin stress fibers and focal adhesions in cultured cells: possible involvement of the rho signal cascade. Cell Struct. Funct. 21, 317–326 (1996).
Liu, B. P., Chrzanowska-Wodnicka, M. & Burridge, K. Microtubule depolymerization induces stress fibers, focal adhesions, and DNA synthesis via the GTP-binding protein Rho. Cell Adhes. Commun. 5, 249–255 (1998).
Pletjushkina, O. J. et al. Maturation of cell–substratum focal adhesions induced by depolymerization of microtubules is mediated by increased cortical tension. Cell Adhes. Commun. 5, 121–135 (1998).
Krylyshkina, O. et al. Modulation of substrate adhesion dynamics via microtubule targeting requires kinesin-1. J. Cell Biol. 156, 349–360 (2002).
Danowski, B. A. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J. Cell Sci. 93, 255–266 (1989).
Kaverina, I., Krylyshkina, O. & Small, J. V. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J. Cell Biol. 146, 1033–1044 (1999).
Kaverina, I., Rottner, K. & Small, J. V. Targeting, capture, and stabilization of microtubules at early focal adhesions. J. Cell Biol. 142, 181–190 (1998).
Ishizaki, T. et al. Coordination of microtubules and the actin cytoskeleton by the Rho effector mDia1. Nature Cell Biol. 3, 8–14 (2001).
Palazzo, A. F., Cook, T. A., Alberts, A. S. & Gundersen, G. G. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nature Cell Biol. 3, 723–729 (2001).
Suter, D. M., Errante, L. D., Belotserkovsky, V. & Forscher, P. The Ig superfamily cell adhesion molecule, apCAM, mediates growth cone steering by substrate–cytoskeletal coupling. J. Cell Biol. 141, 227–240 (1998).
Schaefer, A. W., Kabir, N. & Forscher, P. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J. Cell Biol. 158, 139–152 (2002).
Salmon, W. C., Adams, M. C. & Waterman-Storer, C. M. Dual-wavelength fluorescent speckle microscopy reveals coupling of microtubule and actin movements in migrating cells. J. Cell Biol. 158, 31–37 (2002).
Dunn, G. A. in Cell Adhesion and Motility (eds Curtis, A. S. G. & Pitts, J. D.) 409–423 (Cambridge Univ. Press, Cambridge, UK, 1980).
Dunn, G. A. & Zicha, D. Dynamics of fibroblast spreading. J. Cell Sci. 108, 1239–1249 (1995).
Verkhovsky, A. B., Svitkina, T. M. & Borisy, G. G. Self-polarization and directional motility of cytoplasm. Curr. Biol. 9, 11–20 (1999).
Ballestrem, C., Wehrle-Haller, B., Hinz, B. & Imhof, B. A. Actin-dependent lamellipodia formation and microtubule-dependent tail retraction control-directed cell migration. Mol. Biol. Cell 11, 2999–3012 (2000).
Kirschner, M. & Mitchison, T. Beyond self-assembly: from microtubules to morphogenesis. Cell 45, 329–342 (1986).
Kaverina, I., Krylyshkina, O. & Small, J. V. Regulation of substrate adhesion dynamics during cell motility. Int. J. Biochem. Cell Biol. 34, 746–761 (2002).
Wittmann, T. & Waterman-Storer, C. M. Cell motility: can Rho GTPases and microtubules point the way? J. Cell Sci. 114, 3795–3803 (2001).
Krendel, M., Zenke, F. T. & Bokoch, G. M. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nature Cell Biol. 4, 294–301 (2002).
Euteneuer, U. & Schliwa, M. Persistent, directional motility of cells and cytoplasmic fragments in the absence of microtubules. Nature 310, 58–61 (1984).
Keller, H. U., Naef, A. & Zimmermann, A. Effects of colchicine, vinblastine and nocodazole on polarity, motility, chemotaxis and cAMP levels of human polymorphonuclear leukocytes. Exp. Cell Res. 153, 173–185 (1984).
Glasgow, J. E. & Daniele, R. P. Role of microtubules in random cell migration: stabilization of cell polarity. Cell Motil. Cytoskeleton 27, 88–96 (1994).
Sroka, J., von Gunten, M., Dunn, G. A. & Keller, H. U. Phenotype modulation in non-adherent and adherent sublines of Walker carcinosarcoma cells: the role of cell–substratum contacts and microtubules in controlling cell shape, locomotion and cytoskeletal structure. Int. J. Biochem. Cell Biol. 34, 882–899 (2002).
Goode, B. L., Drubin, D. G. & Barnes, G. Functional cooperation between the microtubule and actin cytoskeletons. Curr. Opin. Cell Biol. 12, 63–71 (2000).
Schroer, T. A. Microtubules don and doff their caps: dynamic attachments at plus and minus ends. Curr. Opin. Cell Biol. 13, 92–96 (2001).
Kolodney, M. S. & Wysolmerski, R. B. Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J. Cell Biol. 117, 73–82 (1992).
Brown, R. A., Talas, G., Porter, R. A., McGrouther, D. A. & Eastwood, M. Balanced mechanical forces and microtubule contribution to fibroblast contraction. J. Cell Physiol. 169, 439–447 (1996).
Lyass, L. A., Bershadsky, A. D., Vasiliev, J. M. & Gelfand, I. M. Microtubule-dependent effect of phorbol ester on the contractility of cytoskeleton of cultured fibroblasts. Proc. Natl Acad. Sci. USA 85, 9538–9541 (1988).
Solomon, F. & Magendantz, M. Cytochalasin separates microtubule disassembly from loss of asymmetric morphology. J. Cell Biol. 89, 157–161 (1981).
Canman, J. C. & Bement, W. M. Microtubules suppress actomyosin-based cortical flow in Xenopus oocytes. J. Cell Sci. 110, 1907–1917 (1997).
Kolodney, M. S. & Elson, E. L. Contraction due to microtubule disruption is associated with increased phosphorylation of myosin regulatory light chain. Proc. Natl Acad. Sci. USA 92, 10252–10256 (1995).
Bornens, M., Paintrand, M. & Celati, C. The cortical microfilament system of lymphoblasts displays a periodic oscillatory activity in the absence of microtubules: implications for cell polarity. J. Cell Biol. 109, 1071–1083 (1989).
Pletjushkina, O. J. et al. Induction of cortical oscillations in spreading cells by depolymerization of microtubules. Cell Motil. Cytoskeleton 48, 235–244 (2001).
Lampidis, T. J., Kolonias, D., Savaraj, N. & Rubin, R. W. Cardiostimulatory and antiarrhythmic activity of tubulin-binding agents. Proc. Natl Acad. Sci. USA 89, 1256–1260 (1992).
Paul, R. J., Bowman, P. S. & Kolodney, M. S. Effects of microtubule disruption on force, velocity, stiffness and [Ca2+](i) in porcine coronary arteries. Am. J. Physiol. Heart Circ. Physiol. 279, H2493–H2501 (2000).
Zhang, D., Jin, N., Rhoades, R. A., Yancey, K. W. & Swartz, D. R. Influence of microtubules on vascular smooth muscle contraction. J. Muscle Res. Cell Motil. 21, 293–300 (2000).
Koide, M. et al. Microtubule depolymerization normalizes in vivo myocardial contractile function in dogs with pressure-overload left ventricular hypertrophy. Circulation 102, 1045–1052 (2000).
Wang, N. et al. Mechanical behavior in living cells consistent with the tensegrity model. Proc. Natl Acad. Sci. USA 98, 7765–7770 (2001).
Ingber, D. E. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J. Cell Sci. 104, 613–627 (1993).
Elbaum, M., Kuchnir Fygenson, D. & Libchaber, A. Buckling microtubules in vesicles. Phys. Rev. Lett. 76, 4078–4081 (1996).
Felgner, H., Frank, R. & Schliwa, M. Flexural rigidity of microtubules measured with the use of optical tweezers. J. Cell Sci. 109, 509–516 (1996).
Elbaum, M., Chausovsky, A., Levy, E. T., Shtutman, M. & Bershadsky, A. D. Microtubule involvement in regulating cell contractility and adhesion-dependent signalling: a possible mechanism for polarization of cell motility. Biochem. Soc. Symp. 65, 147–172 (1999).
Ren, Y., Li, R., Zheng, Y. & Busch, H. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J. Biol. Chem. 273, 34954–34960 (1998).
van Horck, F. P., Ahmadian, M. R., Haeusler, L. C., Moolenaar, W. H. & Kranenburg, O. Characterization of p190RhoGEF, a RhoA-specific guanine nucleotide exchange factor that interacts with microtubules. J. Biol. Chem. 276, 4948–4956 (2001).
Ren, X. D., Kiosses, W. B. & Schwartz, M. A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578–585 (1999).
Fukata, Y., Amano, M. & Kaibuchi, K. Rho–Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol. Sci. 22, 32–39 (2001).
Bershadsky, A. D., Vaisberg, E. A. & Vasiliev, J. M. Pseudopodial activity at the active edge of migrating fibroblast is decreased after drug-induced microtubule depolymerization. Cell Motil. Cytoskeleton 19, 152–158 (1991).
Dunn, G. A., Zicha, D. & Fraylich, P. E. Rapid, microtubule-dependent fluctuations of the cell margin. J. Cell Sci. 110, 3091–3098 (1997).
Waterman-Storer, C. M., Worthylake, R. A., Liu, B. P., Burridge, K. & Salmon, E. D. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nature Cell Biol. 1, 45–50 (1999).
Bretscher, M. S. & Aguado-Velasco, C. Membrane traffic during cell locomotion. Curr. Opin. Cell Biol. 10, 537–541 (1998).
Toomre, D., Keller, P., White, J., Olivo, J. C. & Simons, K. Dual-color visualization of trans-Golgi network to plasma membrane traffic along microtubules in living cells. J. Cell Sci. 112, 21–33 (1999).
Bershadsky, A. D. & Futerman, A. H. Disruption of the Golgi apparatus by brefeldin A blocks cell polarization and inhibits directed cell migration. Proc. Natl Acad. Sci. USA 91, 5686–5689 (1994).
Rodionov, V. I. et al. Microtubule-dependent control of cell shape and pseudopodial activity is inhibited by the antibody to kinesin motor domain. J. Cell Biol. 123, 1811–1820 (1993).
Acknowledgements
We thank O. Krylyskina and G. Resch for their invaluable help with the videos and animation. A.B. and B.G. are grateful to D. Riveline and J. Kirchner for providing the experimental data for figures 2 and 3. A. Huttenlocher is acknowledged for providing the DsRed zyxin construct that was used in figure 5. This work was supported in part by a grant from the Austrian Science Research Council to J.V.S. and I.K. B.G. is the incumbent of the E. Neter Chair in Cell and Tumor Biology; A.B. holds the J. Moss Chair of Biomedical Research.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Online Video 1
A time-lapse video of a goldfish fibroblast that was expressing green fluorescent protein (GFP)–actin (green) and that was also micro-injected with rhodamine-tagged vinculin (red) to mark adhesion complexes (corresponding to Fig. 1). The inset movies correspond to the boxed areas I and IV in Fig. 1. Video 1.1 corresponds to boxed area I, which is shown in Fig. 1b,c and Fig. 2a, and shows newly forming adhesions. Video 1.2 corresponds to the boxed area IV in Fig. 2c and shows sliding, trailing adhesions. The time between frame-pairs in the Quick-Time videos is 60 s for video 1 and 30 s for videos 1.1 and 1.2; the interval between the sequential frames in the GFP and rhodamine channels is 1 s. The online video was provided by O. Krylyshkina. (MOV 128 kb)
Online Video 1.1
Video 1.1 corresponds to boxed area I, which is shown in Fig. 1b,c and Fig. 2a, and shows newly forming adhesions. (MOV 4046 kb)
Online Video 1.2
Video 1.2 corresponds to the boxed area IV in Fig. 2c and shows sliding, trailing adhesions. The time between frame-pairs in the Quick-Time videos is 60 s for video 1 and 30 s for videos 1.1 and 1.2; the interval between the sequential frames in the GFP and rhodamine channels is 1 s. (MOV 5598 kb)
Online Video 2
The targeting of substrate adhesion complexes by microtubules is shown (corresponding to Fig. 5). The video shows a peripheral region of a goldfish fibroblast thatwas transfected with green fluorescent protein–tubulin (green) and DsRed–zyxin (red). The time between frame-pairs was 10 s, with 1 s between the two fluorescent channels in one pair. The targeting events are circled in arrested frames and the inset movies show specific details. Video 2.1 highlights the multiple targeting of one focal adhesion by several microtubules (circled events), as well as the re-routing of one microtubule away from this adhesion to more peripheral adhesions (asterisk). Video 2.2 shows the dissolution of two focal adhesions (circled) after several targeting events. (MOV 513 kb)
Online Video 2.1
Video 2.1 highlights the multiple targeting of one focal adhesion by several microtubules (circled events), as well as the re-routing of one microtubule away from this adhesion to more peripheral adhesions (asterisk). (MOV 11190 kb)
Online Video 2.2
Video 2.2 shows the dissolution of two focal adhesions (circled) after several targeting events. (MOV 5095 kb)
Online Video 3
An animation depicting the salient features of the interactions between microtubules and sites of substrate adhesion. In a migrating cell, protrusion is supported by the formation of focal complexes at the cell front, which can then develop into focal adhesions. the cell rear is limited by focal adhesions that originate through the retraction of former regions of protrusion. Targeting of focal adhesions by microtubules is presumed to be associated with the transmission of a signal (orange stars) that promotes events leading to adhesion disassembly. Targeting of focal adhesions behind the cell front is required to promote adhesion turnover and facilitate further protrusion. Targeting of focal adhesions at the rear is more frequent and is required to promote adhesion release during retraction. The animation programme does not allow a realistic simulation of microtubule dynamics. Provided by G. Resch, Austrian Academy of Science, Austria. (MOV 6402 kb)
41580_2002_BFnrm971_MOESM8_ESM.jpg
Online figure 1 | Microtubule disruption induces stress fibre growth. The activation of actin stress-fibre assembly (right panels) in starved fibroblasts after the initiation of microtubule depolymerization by nocodazole (left panels) is shown. Reproduced with permission from Ref. 25 © (2002) Elsevier Science. (JPG 43 kb)
41580_2002_BFnrm971_MOESM9_ESM.jpg
Online figure 2 | Repetitive targeting of peripheral focal adhesins precedes cell-edge retraction. An example of the multiple targeting of a peripheral adhesion site by microtubules that leads to adhesion translocation and detachment. The panels show video sequences from the periphery of a goldfish fibroblast that was injected with Cy-3-tagged tubulin and rhodamine-tagged vinculin. Times are in minutes and seconds. Different adhesion sites are targeted at different time points, and the frames were selected for the targeting events of one adhesion. Reproduced with permission from Ref. 31 © (2002) The Rockefeller University Press. (JPG 23 kb)
41580_2002_BFnrm971_MOESM10_ESM.jpg
Online figure 3 | Microtubule polymerization dynamics are influenced by mechanical stress in the actin cytoskeleton. The feedback regulation of microtubules by the actin cytoskeleton. Transient relaxation of peripheral contractility induces microtubule depolymerization. A fish fibroblast that was transfected with green fluorescent protein–tubulin (green) and micro-injected with rhodamine-tagged vinculin (red) was exposed for 2–3 min on one edge to the myosin relaxant, ML-7, which was delivered through a micropipette. The first two panels show images before and after application. After removal of the drug (recovery), microtubules repolymerize to the cell edge and target adhesion sites (panel 3). Modified with permission from Ref. 19 © (2002) The Company of Biologists Ltd. (JPG 26 kb)
Related links
Rights and permissions
About this article
Cite this article
Small, J., Geiger, B., Kaverina, I. et al. How do microtubules guide migrating cells?. Nat Rev Mol Cell Biol 3, 957–964 (2002). https://doi.org/10.1038/nrm971
Issue Date:
DOI: https://doi.org/10.1038/nrm971
This article is cited by
-
Complexity of progranulin mechanisms of action in mesothelioma
Journal of Experimental & Clinical Cancer Research (2022)
-
DT-13 inhibits breast cancer cell migration via non-muscle myosin II-A regulation in tumor microenvironment synchronized adaptations
Clinical and Translational Oncology (2020)
-
In vitro wound healing of tumor cells: inhibition of cell migration by selected cytotoxic alkaloids
BMC Pharmacology and Toxicology (2019)
-
The small GTPase RhoG regulates microtubule-mediated focal adhesion disassembly
Scientific Reports (2019)
-
A mechano-signalling network linking microtubules, myosin IIA filaments and integrin-based adhesions
Nature Materials (2019)