Abstract
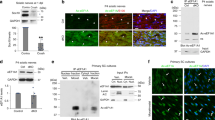
Histone deacetylase 1 (HDAC1) is a nuclear enzyme involved in transcriptional repression. We detected cytosolic HDAC1 in damaged axons in brains of humans with multiple sclerosis and of mice with cuprizone-induced demyelination, in ex vivo models of demyelination and in cultured neurons exposed to glutamate and tumor necrosis factor-α. Nuclear export of HDAC1 was mediated by the interaction with the nuclear receptor CRM-1 and led to impaired mitochondrial transport. The formation of complexes between exported HDAC1 and members of the kinesin family of motor proteins hindered the interaction with cargo molecules, thereby inhibiting mitochondrial movement and inducing localized beading. This effect was prevented by inhibiting HDAC1 nuclear export with leptomycin B, treating neurons with pharmacological inhibitors of HDAC activity or silencing HDAC1 but not other HDAC isoforms. Together these data identify nuclear export of HDAC1 as a critical event for impaired mitochondrial transport in damaged neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Galvin, J.E., Uryu, K., Lee, V.M. & Trojanowski, J.Q. Axon pathology in Parkinson's disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein. Proc. Natl. Acad. Sci. USA 96, 13450–13455 (1999).
Ferguson, B., Matyszak, M.K., Esiri, M.M. & Perry, V.H. Axonal damage in acute multiple sclerosis lesions. Brain 120, 393–399 (1997).
Trapp, B.D. et al. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 338, 278–285 (1998).
Dutta, R. & Trapp, B.D. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology 68, S22–S31; discussion S43–S54 (2007).
Trapp, B.D. & Stys, P.K. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 8, 280–291 (2009).
Beirowski, B. et al. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 6, 6 (2005).
Fisher, E. et al. Imaging correlates of axonal swelling in chronic multiple sclerosis brains. Ann. Neurol. 62, 219–228 (2007).
Stokin, G.B. et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science 307, 1282–1288 (2005).
Watson, D.F., Fittro, K.P., Hoffman, P.N. & Griffin, J.W. Phosphorylation-related immunoreactivity and the rate of transport of neurofilaments in chronic 2,5-hexanedione intoxication. Brain Res. 539, 103–109 (1991).
Yabe, J.T., Pimenta, A. & Shea, T.B. Kinesin-mediated transport of neurofilament protein oligomers in growing axons. J. Cell Sci. 112, 3799–3814 (1999).
Pant, H.C. Dephosphorylation of neurofilament proteins enhances their susceptibility to degradation by calpain. Biochem. J. 256, 665–668 (1988).
Perez-Olle, R. et al. Mutations in the neurofilament light gene linked to Charcot-Marie-Tooth disease cause defects in transport. J. Neurochem. 93, 861–874 (2005).
Kiaei, M. et al. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener. Dis. 2, 246–254 (2005).
Song, S.K. et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26, 132–140 (2005).
Wegner, C., Esiri, M.M., Chance, S.A., Palace, J. & Matthews, P.M. Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology 67, 960–967 (2006).
Stone, J.R. et al. Impaired axonal transport and altered axolemmal permeability occur in distinct populations of damaged axons following traumatic brain injury. Exp. Neurol. 190, 59–69 (2004).
Bitsch, A., Schuchardt, J., Bunkowski, S., Kuhlmann, T. & Bruck, W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain 123, 1174–1183 (2000).
King, A.E. et al. Excitotoxicity mediated by non-NMDA receptors causes distal axonopathy in long-term cultured spinal motor neurons. Eur. J. Neurosci. 26, 2151–2159 (2007).
Williamson, T.L. & Cleveland, D.W. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat. Neurosci. 2, 50–56 (1999).
Adams, J.H., Graham, D.I., Gennarelli, T.A. & Maxwell, W.L. Diffuse axonal injury in non-missile head injury. J. Neurol. Neurosurg. Psychiatry 54, 481–483 (1991).
de Ruijter, A.J., van Gennip, A.H., Caron, H.N., Kemp, S. & van Kuilenburg, A.B. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem. J. 370, 737–749 (2003).
Yang, X.J. & Gregoire, S. Class II histone deacetylases: from sequence to function, regulation, and clinical implication. Mol. Cell. Biol. 25, 2873–2884 (2005).
Yao, Y.L., Yang, W.M. & Seto, E. Regulation of transcription factor YY1 by acetylation and deacetylation. Mol. Cell. Biol. 21, 5979–5991 (2001).
Chen, Z. et al. Induction and superinduction of growth arrest and DNA damage gene 45 (GADD45) alpha and beta messenger RNAs by histone deacetylase inhibitors trichostatin A (TSA) and butyrate in SW620 human colon carcinoma cells. Cancer Lett. 188, 127–140 (2002).
Hubbert, C. et al. HDAC6 is a microtubule-associated deacetylase. Nature 417, 455–458 (2002).
Chawla, S., Vanhoutte, P., Arnold, F.J., Huang, C.L. & Bading, H. Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J. Neurochem. 85, 151–159 (2003).
Kawaguchi, Y. et al. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 115, 727–738 (2003).
Dompierre, J.P. et al. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. J. Neurosci. 27, 3571–3583 (2007).
Schumacher, P.A., Eubanks, J.H. & Fehlings, M.G. Increased calpain I-mediated proteolysis, and preferential loss of dephosphorylated NF200, following traumatic spinal cord injury. Neuroscience 91, 733–744 (1999).
Pitt, D., Werner, P. & Raine, C.S. Glutamate excitotoxicity in a model of multiple sclerosis. Nat. Med. 6, 67–70 (2000).
Shen, S. et al. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat. Neurosci. 11, 1024–1034 (2008).
Li, J. et al. Inhibition of p53 transcriptional activity: a potential target for future development of therapeutic strategies for primary demyelination. J. Neurosci. 28, 6118–6127 (2008).
Birgbauer, E., Rao, T.S. & Webb, M. Lysolecithin induces demyelination in vitro in a cerebellar slice culture system. J. Neurosci. Res. 78, 157–166 (2004).
Bruck, W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J. Neurol. 252 (suppl. 5), v3–v9 (2005).
Ouardouz, M. et al. Glutamate receptors on myelinated spinal cord axons: II. AMPA and GluR5 receptors. Ann. Neurol. 65, 160–166 (2009).
Kurnellas, M.P., Donahue, K.C. & Elkabes, S. Mechanisms of neuronal damage in multiple sclerosis and its animal models: role of calcium pumps and exchangers. Biochem. Soc. Trans. 35, 923–926 (2007).
Wen, W., Meinkoth, J.L., Tsien, R.Y. & Taylor, S.S. Identification of a signal for rapid export of proteins from the nucleus. Cell 82, 463–473 (1995).
Wolff, B., Sanglier, J.J. & Wang, Y. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem. Biol. 4, 139–147 (1997).
Uo, T., Veenstra, T.D. & Morrison, R.S. Histone deacetylase inhibitors prevent p53-dependent and p53-independent Bax-mediated neuronal apoptosis through two distinct mechanisms. J. Neurosci. 29, 2824–2832 (2009).
Dasmahapatra, G., Almenara, J.A. & Grant, S. Flavopiridol and histone deacetylase inhibitors promote mitochondrial injury and cell death in human leukemia cells that overexpress Bcl-2. Mol. Pharmacol. 69, 288–298 (2006).
Morrison, B.E. et al. Neuroprotection by histone deacetylase-related protein. Mol. Cell. Biol. 26, 3550–3564 (2006).
Ballas, N. et al. Regulation of neuronal traits by a novel transcriptional complex. Neuron 31, 353–365 (2001).
Zhang, Y. et al. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 22, 1168–1179 (2003).
Bolger, T.A. & Yao, T.P. Intracellular trafficking of histone deacetylase 4 regulates neuronal cell death. J. Neurosci. 25, 9544–9553 (2005).
Hoshino, M. et al. Histone deacetylase activity is retained in primary neurons expressing mutant huntingtin protein. J. Neurochem. 87, 257–267 (2003).
Morfini, G.A. et al. Axonal transport defects in neurodegenerative diseases. J. Neurosci. 29, 12776–12786 (2009).
Gu, H., Liang, Y., Mandel, G. & Roizman, B. Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc. Natl. Acad. Sci. USA 102, 7571–7576 (2005).
Zhang, Y. & Jones, C. The bovine herpesvirus 1 immediate-early protein (bICP0) associates with histone deacetylase 1 to activate transcription. J. Virol. 75, 9571–9578 (2001).
Viatour, P. et al. Cytoplasmic IkappaBalpha increases NF-kappaB-independent transcription through binding to histone deacetylase (HDAC) 1 and HDAC3. J. Biol. Chem. 278, 46541–46548 (2003).
Acknowledgements
We thank the University of California Los Angeles Brain Bank and the UK MS Tissue Bank at Imperial College of London for providing the multiple sclerosis tissue samples, C. Seiser (University of Vienna) for providing the antibodies to the N-terminal domain of HDAC1, R. Bansal (University of Connecticut Health Science Center at Farmington) for anti-O4 hybridoma supernatant, X. Pedre and J. Li for rodent handling, S. Schreiber (Broad Institute, Massachusetts Institute of Technology) for tubacin, E. Nestler and H. Covington (Mount Sinai School of Medicine) for MS-275, S. Lagger for sharing unpublished results and critical comments on the manuscript, P. Lobel for discussions regarding MALDI-TOF data, J. Zheng and K. Teng for helpful discussion and R. Rosa for the support of the New Jersey Multiple Sclerosis Research Foundation. The study was supported by NIH-RO1 NS-42925 (P.C.) and NMSS RG-3957. J.Y.K.'s salary was supported in part also by grant no. 07-3203-BIR-E-0 from the New Jersey Commission on Traumatic Brain injury and CB1-0704-2 from the Christopher and Dana Reeve Foundation to P.C.
Author information
Authors and Affiliations
Contributions
The majority of the experiments in the manuscript were conducted by J.Y.K. S.S. performed the first experiments in the demyelinated mice and the first mass spectrometry. K.D. performed the in vivo confocal analysis in demyelinated mouse brains and the SAPK experiments; O.H. and R.R. performed the analysis of the human material; Y.H. performed the qt-PCR experiments. P.C. designed the experimental plan, supervised the project and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Table 1 (PDF 2802 kb)
Rights and permissions
About this article
Cite this article
Kim, J., Shen, S., Dietz, K. et al. HDAC1 nuclear export induced by pathological conditions is essential for the onset of axonal damage. Nat Neurosci 13, 180–189 (2010). https://doi.org/10.1038/nn.2471
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2471
This article is cited by
-
The role of HDAC3 and its inhibitors in regulation of oxidative stress and chronic diseases
Cell Death Discovery (2023)
-
Transcriptional mutagenesis of α-synuclein caused by DNA oxidation in Parkinson’s disease pathogenesis
Acta Neuropathologica (2023)
-
Trait − driven analysis of the 2p15p16.1 microdeletion syndrome suggests a complex pattern of interactions between candidate genes
Genes & Genomics (2023)
-
EGFR phosphorylates HDAC1 to regulate its expression and anti-apoptotic function
Cell Death & Disease (2021)
-
HDAC1 deregulation promotes neuronal loss and deficit of motor function in stroke pathogenesis
Scientific Reports (2021)