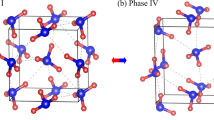
Abstract
Ammonia is an important compound with many uses, such as in the manufacture of fertilizers, explosives and pharmaceuticals. As an archetypal hydrogen-bonded system, the properties of ammonia under pressure are of fundamental interest, and compressed ammonia has a significant role in planetary physics. We predict new high-pressure crystalline phases of ammonia (NH3) through a computational search based on first-principles density-functional-theory calculations1. Ammonia is known to form hydrogen-bonded solids2,3,4,5,6, but we predict that at higher pressures it will form ammonium amide ionic solids consisting of alternate layers of NH4+ and NH2− ions. These ionic phases are predicted to be stable over a wide range of pressures readily obtainable in laboratory experiments. The occurrence of ionic phases is rationalized in terms of the relative ease of forming ammonium and amide ions from ammonia molecules, and the volume reduction on doing so. We also predict that the ionic bonding cannot be sustained under extreme compression and that, at pressures beyond the reach of current static-loading experiments, ammonia will return to hydrogen-bonded structures consisting of neutral NH3 molecules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Martin, R. M. Electronic Structure: Basic Theory and Practical Methods (Cambridge University Press, Cambridge, 2004).
Reed, J. W. & Harris, P. M. Neutron diffraction study of solid deuteroammonia. J. Chem. Phys. 35, 1730–1737 (1961).
Hewat, A. W. & Riekel, C. The crystal structure of deuteroammonia between 2 and 180 K by neutron powder profile refinement. Acta Crystallogr. A A35, 569–571 (1979).
Leclerq, F., Damay, P. & Foukani, M. Structure of powder deuteroammonia between 2 and 180 K revisited: A refinement of the neutron diffraction pattern taking into account molecular reorientations: analysis of the diffuse intensity. J. Chem. Phys. 102, 4400–4408 (1995).
Loveday, J. S. et al. Structure of deuterated ammonia IV. Phys. Rev. Lett. 76, 74–77 (1996).
Datchi, F. et al. Solid ammonia at high pressure: A single-crystal x-ray diffraction study to 123 GPa. Phys. Rev. B 73, 174111 (2006).
Nelson, D. D. Jr., Fraser, G. T. & Klemperer, W. Does ammonia hydrogen bond? Science 238, 1670–1674 (1987).
Hubbard, W. B. Interiors of the giant planets. Science 214, 145–149 (1981).
Sasselov, D. D. Extrasolar planets. Nature 451, 29–31 (2008).
Cavazzoni, C. et al. Superionic and metallic states of water and ammonia at giant planet conditions. Science 283, 44–46 (1999).
Gauthier, M., Pruzan, Ph., Chervin, J. C. & Besson, J. M. Raman scattering study of ammonia up to 75 GPa: Evidence for bond symmetrization at 60 GPa. Phys. Rev. B 37, 2102–2115 (1988).
Ninet, S., Datchi, F., Saitta, A. M., Lazzeri, M. & Canny, B. Raman spectrum of ammonia IV. Phys. Rev. B 74, 104101 (2006).
Gauthier, M., Pruzan, Ph., Chervin, J. C. & Polian, A. Brillouin study of liquid and solid ammonia up to 20 GPa. Solid State Commun. 68, 149–153 (1988).
Sakashita, M., Yamawaki, H., Fujihisa, H. & Aoki, K. Phase study of NH3 to 100 GPa by infrared absorption. Rev. High Pressure Sci. Technol. 7, 796–798 (1998).
Kamb, B. & Davis, B. L. Ice VII, the densest form of ice. Proc. Natl Acad. Sci. USA 52, 1433–1439 (1964).
Struzhkin, V. V., Goncharov, A. F., Hemley, R. J. & Mao, H.-K. Cascading Fermi resonances and the soft mode in dense ice. Phys. Rev. Lett. 78, 4446–4449 (1997).
Goncharov, A. F., Struzhkin, V. V., Mao, H.-K. & Hemley, R. J. Raman spectroscopy of dense H2O and the transition to symmetric hydrogen bonds. Phys. Rev. Lett. 83, 1998–2001 (1999).
Aoki, K., Yamawaki, H. & Sakashita, M. Observation of Fano interference in high-pressure ice VII. Phys. Rev. Lett. 76, 784–786 (1996).
Fortes, A. D., Brodholt, J. P., Wood, I. G. & Voc˘adlo, L. Hydrogen bonding in solid ammonia from ab initio calculations. J. Chem. Phys. 118, 5987–5994 (2003).
Pickard, C. J. & Needs, R. J. High pressure phases of silane. Phys. Rev. Lett. 97, 045504 (2006).
Pickard, C. J. & Needs, R. J. Structure of phase III of hydrogen. Nature Phys. 3, 473–476 (2007).
Pickard, C. J. & Needs, R. J. Metallization of aluminum hydride at high pressures: A first-principles study. Phys. Rev. B 76, 144114 (2007).
Pickard, C. J. & Needs, R. J. When is H2O not water? J. Chem. Phys. 127, 244503 (2007).
Eremets, M. I., Trojan, I. A., Medvedev, S. A., Tse, J. S. & Yao, Y. Superconductivity in hydrogen dominant materials: silane. Science 319, 1506–1509 (2008).
Goncharenko, I. et al. Pressure-induced hydrogen-dominant metallic state in aluminum hydride. Phys. Rev. Lett. 100, 045504 (2008).
Segall, M. D., Shah, R., Pickard, C. J. & Payne, M. C. Population analysis of plane-wave electronic structure calculations of bulk materials. Phys. Rev. B 54, 16317–16320 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Liebman, J. F. Existence and estimated enthalpies of formation of ammonium hydroxide, hydronium amide, and some related species. Struct. Chem. 8, 313–315 (1997).
Somayazulu, M. et al. Novel broken symmetry phase from N2O at high pressures and high temperatures. Phys. Rev. Lett. 87, 135504 (2001).
Meng, Y. et al. Hard x-ray radiation induced dissociation of N2 and O2 molecules and the formation of ionic nitrogen oxide phases under pressure. Phys. Rev. B 74, 214107 (2006).
Fortes, A. D., Brodholt, J. P., Wood, I. G., Voc˘adlo, L. & Jenkins, H. D. B. Ab initio simulation of ammonia monohydrate (NH3·H2O) and ammonium hydroxide (NH4OH). J. Chem. Phys. 115, 7006–7014 (2001).
Clark, S. J. et al. First principles methods using CASTEP. Z. Kristallogr. 220, 567–570 (2005).
Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 41, 7892–7895 (1990).
Acknowledgements
We thank S. Clark for help with the Raman calculations. R.J.N. was supported by the Engineering and Physical Sciences Research Council (EPSRC) of the UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
C.J.P. is an author of the CASTEP code, used in this work and sold commercially by Accelrys.
Supplementary information
Supplementary Information
Supplementary Information (PDF 3034 kb)
Rights and permissions
About this article
Cite this article
Pickard, C., Needs, R. Highly compressed ammonia forms an ionic crystal. Nature Mater 7, 775–779 (2008). https://doi.org/10.1038/nmat2261
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2261
This article is cited by
-
First-principles search of hot superconductivity in La-X-H ternary hydrides
npj Computational Materials (2022)
-
Study of disorder in pulsed laser deposited double perovskite oxides by first-principle structure prediction
npj Computational Materials (2021)
-
First principles study of dense and metallic nitric sulfur hydrides
Communications Chemistry (2021)
-
Formation of ammonia–helium compounds at high pressure
Nature Communications (2020)
-
Chemistry under high pressure
Nature Reviews Chemistry (2020)