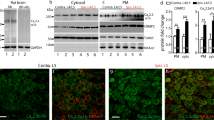
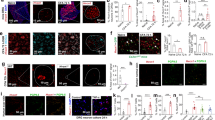
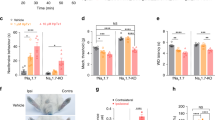
Abstract
The use of N-type voltage-gated calcium channel (CaV2.2) blockers to treat pain is limited by many physiological side effects. Here we report that inflammatory and neuropathic hypersensitivity can be suppressed by inhibiting the binding of collapsin response mediator protein 2 (CRMP-2) to CaV2.2 and thereby reducing channel function. A peptide of CRMP-2 fused to the HIV transactivator of transcription (TAT) protein (TAT-CBD3) decreased neuropeptide release from sensory neurons and excitatory synaptic transmission in dorsal horn neurons, reduced meningeal blood flow, reduced nocifensive behavior induced by formalin injection or corneal capsaicin application and reversed neuropathic hypersensitivity produced by an antiretroviral drug. TAT-CBD3 was mildly anxiolytic without affecting memory retrieval, sensorimotor function or depression. At doses tenfold higher than that required to reduce hypersensitivity in vivo, TAT-CBD3 caused a transient episode of tail kinking and body contortion. By preventing CRMP-2–mediated enhancement of CaV2.2 function, TAT-CBD3 alleviated inflammatory and neuropathic hypersensitivity, an approach that may prove useful in managing chronic pain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Staats, P.S. et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. J. Am. Med. Assoc. 291, 63–70 (2004).
Rauck, R.L. et al. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J. Pain Symptom Manage. 31, 393–406 (2006).
Wallace, M.S. Intrathecal ziconotide for severe chronic pain: safety and tolerability results of an open-label, long-term trial. Anesth. Analg. 106, 628–637 (2008).
Schmidtko, A., Lotsch, J., Freynhagen, R. & Geisslinger, G. Ziconotide for treatment of severe chronic pain. Lancet 375, 1569–1577 (2010).
Brittain, J.M. et al. An atypical role for collapsin response mediator protein 2 (CRMP-2) in neurotransmitter release via interaction with presynaptic voltage-gated Ca2+ channels. J. Biol. Chem. 284, 31375–31390 (2009).
Chi, X.X. et al. Regulation of N-type voltage-gated calcium (CaV2.2) channels and transmitter release by collapsin response mediator protein-2 (CRMP-2) in sensory neurons. J. Cell Sci. 122, 4351–4362 (2009).
Goshima, Y., Nakamura, F., Strittmatter, P. & Strittmatter, S.M. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature 376, 509–514 (1995).
Inagaki, N. et al. CRMP-2 induces axons in cultured hippocampal neurons. Nat. Neurosci. 4, 781–782 (2001).
Yoshimura, T. et al. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 120, 137–149 (2005).
Morita, T. & Sobue, K. Specification of neuronal polarity regulated by local translation of CRMP2 and Tau via the mTOR-p70S6K pathway. J. Biol. Chem. 284, 27734–27745 (2009).
Arimura, N. et al. CRMP-2 directly binds to cytoplasmic dynein and interferes with its activity. J. Neurochem. 111, 380–390 (2009).
Fukata, Y. et al. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat. Cell Biol. 4, 583–591 (2002).
Wang, Y. et al. In silico docking and electrophysiological characterization of lacosamide binding sites on collapsin response mediator protein 2 (CRMP-2) identifies a pocket important in modulating sodium channel slow inactivation. J. Biol. Chem. 285, 25296–25307 (2010).
Schwarze, S.R., Ho, A., Vocero-Akbani, A. & Dowdy, S.F. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285, 1569–1572 (1999).
Andrade, A., Denome, S., Jiang, Y.Q., Marangoudakis, S. & Lipscombe, D. Opioid inhibition of N-type Ca2+ channels and spinal analgesia couple to alternative splicing. Nat. Neurosci. 13, 1249–1256 (2010).
Todd, A.J. Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci. 11, 823–836 (2010).
Liu, T., Xu, Z.Z., Park, C.K., Berta, T. & Ji, R.R. Toll-like receptor 7 mediates pruritus. Nat. Neurosci. 13, 1460–1462 (2010).
Yamamoto, T. & Takahara, A. Recent updates of N-type calcium channel blockers with therapeutic potential for neuropathic pain and stroke. Curr. Top. Med. Chem. 9, 377–395 (2009).
Zamponi, G.W., Lewis, R.J., Todorovic, S.M., Arneric, S.P. & Snutch, T.P. Role of voltage-gated calcium channels in ascending pain pathways. Brain Res. Rev. 60, 84–89 (2009).
Catterall, W.A. & Few, A.P. Calcium channel regulation and presynaptic plasticity. Neuron 59, 882–901 (2008).
Dodge, F.A. Jr. & Rahamimoff, R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J. Physiol. (Lond.) 193, 419–432 (1967).
Patapoutian, A., Tate, S. & Woolf, C.J. Transient receptor potential channels: targeting pain at the source. Nat. Rev. Drug Discov. 8, 55–68 (2009).
Goadsby, P.J. Calcitonin gene-related peptide (CGRP) antagonists and migraine: is this a new era? Neurology 70, 1300–1301 (2008).
Kunkler, P.E., Ballard, C.J., Oxford, G.S. & Hurley, J.H. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain 152, 38–44 (2010).
Snutch, T.P. Targeting chronic and neuropathic pain: the N-type calcium channel comes of age. NeuroRx 2, 662–670 (2005).
Dubuisson, D. & Dennis, S.G. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 4, 161–174 (1977).
Hunskaar, S. & Hole, K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 30, 103–114 (1987).
Dickenson, A.H. & Sullivan, A.F. Subcutaneous formalin-induced activity of dorsal horn neurones in the rat: differential response to an intrathecal opiate administered pre or post formalin. Pain 30, 349–360 (1987).
Coderre, T.J., Vaccarino, A.L. & Melzack, R. Central nervous system plasticity in the tonic pain response to subcutaneous formalin injection. Brain Res. 535, 155–158 (1990).
Guo, A., Vulchanova, L., Wang, J., Li, X. & Elde, R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur. J. Neurosci. 11, 946–958 (1999).
Nakamura, A. et al. Morphological and immunohistochemical characterization of the trigeminal ganglion neurons innervating the cornea and upper eyelid of the rat. J. Chem. Neuroanat. 34, 95–101 (2007).
Joseph, E.K., Chen, X., Khasar, S.G. & Levine, J.D. Novel mechanism of enhanced nociception in a model of AIDS therapy-induced painful peripheral neuropathy in the rat. Pain 107, 147–158 (2004).
Bhangoo, S.K., Ripsch, M.S., Buchanan, D.J., Miller, R.J. & White, F.A. Increased chemokine signaling in a model of HIV1-associated peripheral neuropathy. Mol. Pain. 5, 48 (2009).
Cai, S.R. et al. The kinetics and tissue distribution of protein transduction in mice. Eur. J. Pharm. Sci. 27, 311–319 (2006).
Hamm, R.J., Pike, B.R., O'Dell, D.M., Lyeth, B.G. & Jenkins, L.W. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J. Neurotrauma 11, 187–196 (1994).
D'Hooge, R. & De Deyn, P.P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Brain Res. Rev. 36, 60–90 (2001).
Gage, F.H., Chen, K.S., Buzsaki, G. & Armstrong, D. Experimental approaches to age-related cognitive impairments. Neurobiol. Aging 9, 645–655 (1988).
Rauck, R.L., Wallace, M.S., Burton, A.W., Kapural, L. & North, J.M. Intrathecal ziconotide for neuropathic pain: a review. Pain Pract. 9, 327–337 (2009).
Bourin, M. & Hascoet, M. The mouse light/dark box test. Eur. J. Pharmacol. 463, 55–65 (2003).
Lalonde, R. & Strazielle, C. Relations between open-field, elevated plus-maze, and emergence tests as displayed by C57/BL6J and BALB/c mice. J. Neurosci. Methods 171, 48–52 (2008).
Varty, G.B., Cohen-Williams, M.E. & Hunter, J.C. The antidepressant-like effects of neurokinin NK1 receptor antagonists in a gerbil tail suspension test. Behav. Pharmacol. 14, 87–95 (2003).
Cryan, J.F., Mombereau, C. & Vassout, A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 29, 571–625 (2005).
Schwarze, S.R. & Dowdy, S.F. In vivo protein transduction: intracellular delivery of biologically active proteins, compounds and DNA. Trends Pharmacol. Sci. 21, 45–48 (2000).
Yoon, J.S. et al. Characteristics of HIV-Tat protein transduction domain. J. Microbiol. 42, 328–335 (2004).
Winquist, R.J., Pan, J.Q. & Gribkoff, V.K. Use-dependent blockade of Cav2.2 voltage-gated calcium channels for neuropathic pain. Biochem. Pharmacol. 70, 489–499 (2005).
Cizkova, D. et al. Localization of N-type Ca2+ channels in the rat spinal cord following chronic constrictive nerve injury. Exp. Brain Res. 147, 456–463 (2002).
Saegusa, H. et al. Suppression of inflammatory and neuropathic pain symptoms in mice lacking the N-type Ca2+ channel. EMBO J. 20, 2349–2356 (2001).
Malmberg, A.B. & Yaksh, T.L. Voltage-sensitive calcium channels in spinal nociceptive processing: blockade of N- and P-type channels inhibits formalin-induced nociception. J. Neurosci. 14, 4882–4890 (1994).
Seward, E., Hammond, C. & Henderson, G. Mu-opioid-receptor-mediated inhibition of the N-type calcium-channel current. Proc. Biol. Soc. 244, 129–135 (1991).
Bell, T.J., Thaler, C., Castiglioni, A.J., Helton, T.D. & Lipscombe, D. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron 41, 127–138 (2004).
Altier, C. et al. Differential role of N-type calcium channel splice isoforms in pain. J. Neurosci. 27, 6363–6373 (2007).
Farazifard, R., Safarpour, F., Sheibani, V. & Javan, M. Eye-wiping test: a sensitive animal model for acute trigeminal pain studies. Brain Res. Brain Res. Protoc. 16, 44–49 (2005).
Le Bars, D., Gozariu, M. & Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 53, 597–652 (2001).
Doak, G.J. & Sawynok, J. Formalin-induced nociceptive behavior and edema: involvement of multiple peripheral 5-hydroxytryptamine receptor subtypes. Neuroscience 80, 939–949 (1997).
Whiteside, G.T., Boulet, J.M. & Walker, K. The role of central and peripheral mu opioid receptors in inflammatory pain and edema: a study using morphine and DiPOA ([8-(3,3-diphenyl-propyl)-4-oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-3-yl]-acetic acid). J. Pharmacol. Exp. Ther. 314, 1234–1240 (2005).
Yarchoan, R. et al. Phase 1 studies of 2′,3′-dideoxycytidine in severe human immunodeficiency virus infection as a single agent and alternating with zidovudine (AZT). Lancet 1, 76–81 (1988).
Dubinsky, R.M., Yarchoan, R., Dalakas, M. & Broder, S. Reversible axonal neuropathy from the treatment of AIDS and related disorders with 2′,3′-dideoxycytidine (ddC). Muscle Nerve 12, 856–860 (1989).
Bhangoo, S.K. et al. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav. Immun. 21, 581–591 (2007).
Nakanishi, O., Ishikawa, T. & Imamura, Y. Modulation of formalin-evoked hyperalgesia by intrathecal N-type Ca channel and protein kinase C inhibitor in the rat. Cell. Mol. Neurobiol. 19, 191–197 (1999).
Acknowledgements
This work is supported by grants from the US National Institutes of Health: Dental and Craniofacial Research (DE14318-06 to J.C.F. and DE017794 to R.-R.J.), Drug Abuse (DA026040 to F.A.W.), Neurological Disorders and Stroke (NS051668 to C.M.H. and NS050131 to N.B.) and Environmental Health Sciences (ES017430 to G.S.O. and J.H.H.); the Indiana State Department of Health−Spinal Cord and Brain Injury Fund (A70-0-079212 to N.B. and A70-9-079138 to R.K.) and the Indiana University Biomedical Committee–Research Support Funds (2286501 to R.K.); a National Scientist Development Grant from the American Heart Association (SDG5280023 to R.K.); and the Elwert Award in Medicine to R.K. J.M.B. is the recipient of a Larry Kays Medical Neuroscience fellowship. S.M.W. is a Stark Scholar. We thank A. Molosh and members of the Pain and Sensory Group for discussions, S.K. Ahuja for assistance with behavioral experiments and C. Kohn for comments on the manuscript.
Author information
Authors and Affiliations
Contributions
J.M.B. performed molecular biology, biochemistry and calcium imaging experiments and analyzed the data. D.B.D. carried out the spinal cord slice release and formalin behavior experiments and helped to write the manuscript. S.M.W. performed immunocytochemistry and wrote the manuscript. C.B. carried out the laser Doppler blood flowmetry. J.H.H. analyzed the blood flow data. P.L.J. and S.D.F. performed anxiety and despair behavior experiments. W.Z. and Y.W. performed DRG and hippocampal patching. C.-K.P. conducted electrophysiology in spinal cord slices. W.X. and X.J. performed electrophysiology on brain slices. B.S.S. carried out the DRG release assays. T.B., N.B. and J.M.B. performed and analyzed the calcium imaging experiments. B.M.C., M.R.D. and M.S.R. performed DRG immunocytochemistry and ddC behavior experiments. M.K. and S.O.M. performed the surface plasmon resonance experiments and analyzed the data. N.L. performed the rotarod and water maze experiments. J.C.F. performed the nocifensive behavior experiments and editing of the manuscript. N.M.A. and A.H. synthesized the peptide blot. X.-M.X., C.M.H., M.R.V., G.S.O. and A.S. contributed to editing of the manuscript. R.-R.J contributed to electrophysiology of spinal cord slices and editing of the manuscript. F.A.W. analyzed the ddC behavior data and contributed to writing and editing the manuscript. R.K. identified the peptide, conceived the study, designed and supervised the overall project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Table 1 and Supplementary Methods (PDF 2807 kb)
Rights and permissions
About this article
Cite this article
Brittain, J., Duarte, D., Wilson, S. et al. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca2+ channel complex. Nat Med 17, 822–829 (2011). https://doi.org/10.1038/nm.2345
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2345
This article is cited by
-
Regulation of N-type calcium channels by nociceptin receptors and its possible role in neurological disorders
Molecular Brain (2022)
-
Non-SUMOylated CRMP2 decreases NaV1.7 currents via the endocytic proteins Numb, Nedd4-2 and Eps15
Molecular Brain (2021)
-
Inhibition of autophagy by CRMP2-derived peptide ST2-104 (R9-CBD3) via a CaMKKβ/AMPK/mTOR pathway contributes to ischemic postconditioning-induced neuroprotection against cerebral ischemia-reperfusion injury
Molecular Brain (2021)
-
Differential expression of Cdk5-phosphorylated CRMP2 following a spared nerve injury
Molecular Brain (2020)
-
Nasal delivery of a CRMP2-derived CBD3 adenovirus improves cognitive function and pathology in APP/PS1 transgenic mice
Molecular Brain (2020)