Abstract
Molecular targeting of protein kinases is a new paradigm in the treatment of cancer. The clinical efficacy of low-molecular weight inhibitors of ABL, stem-cell growth-factor receptor, and the epidermal growth factor receptor in different tumor types is witness to the power of this approach. The presence of activating mutations of a kinase, or an increased gene copy number, might anticipate tumor responsiveness to its targeting. Thyroid cancer is the most prevalent endocrine malignancy and is frequently associated with the oncogenic conversion of two specific protein kinases, RET and BRAF. Small-molecule inhibitors of both kinases have already reached the clinical testing stage. Protein kinases other than RET and BRAF are also being evaluated for their potential in thyroid-cancer treatment.
Key Points
-
Thyroid cancer is the most prevalent endocrine malignancy
-
It is frequently associated with the activation of specific protein kinases
-
There is good evidence that alterations in at least two kinases, RET and BRAF, are causative events in thyroid-cancer initiation
-
Molecular targeting of RET and BRAF holds promise for thyroid-cancer treatment
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
DeLellis RA and Williams ED (2004) Thyroid and parathyroid tumors. In World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs, 51–56 (Eds DeLellis RA et al.) Geneva: WHO Press
Sherman SI (2003) Thyroid carcinoma. Lancet 361: 501–511
Schlumberger MJ (1998) Papillary and follicular thyroid carcinoma. N Engl J Med 338: 297–306
Fagin JA (2004) How thyroid tumors start and why it matters: kinase mutants as targets for solid cancer pharmacotherapy. J Endocrinol 183: 249–256
Xing M (2005) BRAF mutation in thyroid cancer. Endocr Relat Cancer 12: 245–262
Pierotti MA et al. (1996) Cytogenetics and molecular genetics of carcinomas arising from thyroid epithelial follicular cells. Genes Chromosomes Cancer 16: 1–14
Eng C (2003) PTEN: one gene, many syndromes. Hum Mutat 22: 183–198
McIver B et al. (2004) The PAX8/PPARγ fusion oncogene as a potential therapeutic target in follicular thyroid carcinoma. Curr Drug Targets Immune Endocr Metabol Disord 4: 221–234
Ain KB (1999) Anaplastic thyroid carcinoma: a therapeutic challenge. Semin Surg Oncol 16: 64–69
Ordonez N et al. (2004) Undifferentiated (anaplastic) carcinoma. In World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs, 77–80 (Eds DeLellis RA et al.) Geneva: WHO Press
Matias-Guiu X et al. (2004) Medullary thyroid carcinoma. In World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs, 86–91 (Eds DeLellis RA et al.). Geneva: WHO Press
Marx SJ (2005) Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat Rev Cancer 5: 367–375
Wells SA and Nevins JR (2004) Evolving strategies for targeted cancer therapy—past, present, and future. J Natl Cancer Inst 96: 980–981
Skinner MA et al. (2005) Prophylactic thyroidectomy in multiple endocrine neoplasia type 2A. N Engl J Med 353: 1105–1113
Sawyers C (2004) Targeted cancer therapy. Nature 432: 294–297
Dancey J and Sausville EA (2003) Issues and progress with protein kinase inhibitors for cancer treatment. Nat Rev Drug Discov 2: 296–313
Daub H et al. (2004) Strategies to overcome resistance to targeted protein kinase inhibitors. Nat Rev Drug Discov 3: 1001–1010
Pao W and Miller VA (2005) Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: current knowledge and future directions. J Clin Oncol 23: 2556–2568
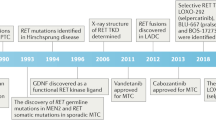
Airaksinen MS and Saarma M (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3: 383–394
Santoro M et al. (2004) Minireview: RET: normal and abnormal functions. Endocrinology 145: 5448–5451
Jijiwa M et al. (2004) A targeting mutation of tyrosine 1062 in Ret causes a marked decrease of enteric neurons and renal hypoplasia. Mol Cell Biol 24: 8026–8036
Melillo RM et al. (2005) The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest 115: 1068–1081
Eng C and Mulligan LM (1997) Mutations of the RET proto-oncogene in the multiple endocrine neoplasia type 2 syndromes, related sporadic tumours, and Hirschsprung disease. Hum Mutat 9: 97–109
Bugalho MJ et al. (2003) Molecular diagnosis of multiple endocrine neoplasia type 2. Expert Rev Mol Diagn 3: 769–779
Kouvaraki MA et al. (2005) RET proto-oncogene: a review and update of genotype–phenotype correlations in hereditary medullary thyroid cancer and associated endocrine tumors. Thyroid 15: 531–544
Carlomagno F et al. (2002) ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res 62: 7284–7290
Carlomagno F et al. (2002) The kinase inhibitor PP1 blocks tumorigenesis induced by RET oncogenes. Cancer Res 62: 1077–1082
Carniti C et al. (2003) PP1 inhibitor induces degradation of RETMEN2A and RETMEN2B oncoproteins through proteosomal targeting. Cancer Res 63: 2234–2243
Carlomagno F et al. (2003) Efficient inhibition of RET/papillary thyroid carcinoma oncogenic kinases by 4-amino-5-(4-chloro-phenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2). J Clin Endocrinol Metab 88: 1897–1902
Strock CJ et al. (2003) CEP-701 and CEP-751 inhibit constitutively activated RET tyrosine kinase activity and block medullary thyroid carcinoma cell growth. Cancer Res 63: 5559–5563
Cuccuru G et al. (2004) Cellular effects and antitumor activity of RET inhibitor RPI-1 on MEN2A-associated medullary thyroid carcinoma. J Natl Cancer Inst 96: 1006–1014
Ezzat S et al. (2005) Dual inhibition of RET and FGFR4 restrains medullary thyroid cancer cell growth. Clin Cancer Res 11: 1336–1341
Ryan AJ and Wedge SR (2005) ZD6474—a novel inhibitor of VEGFR and EGFR tyrosine kinase activity. Br J Cancer 92: S6–S13
Vitagliano D et al. (2004) Regulation of p27Kip1 protein levels contributes to mitogenic effects of the RET/PTC kinase in thyroid carcinoma cells. Cancer Res 64: 3823–3829
Vidal M et al. (2005) ZD6474 suppresses oncogenic RET isoforms in a Drosophila model for type 2 multiple endocrine neoplasia syndromes and papillary thyroid carcinoma. Cancer Res 65: 3538–3541
Carlomagno F et al. (2004) Disease associated mutations at valine 804 in the RET receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene 23: 6056–6063
Lesueur F et al. (2005) Germline homozygous mutations at codon 804 in the RET protooncogene in medullary thyroid carcinoma/multiple endocrine neoplasia type 2A patients. J Clin Endocrinol Metab 90: 3454–3457
Sebolt-Leopold JS and Herrera R (2004) Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer 4: 937–947
Oler G et al. (2005) Investigation of BRAF mutation in a series of papillary thyroid carcinoma and matched-lymph node metastasis reveals a new mutation in metastasis. Clin Endocrinol (Oxf) 62: 509–511
Knauf JA et al. (2005) Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res 65: 4238–4245
Nikiforova MN et al. (2003) BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab 88: 5399–5404
Ciampi R et al. (2005) Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J Clin Invest 115: 94–101
Bollag G et al. (2003) Raf pathway inhibitors in oncology. Curr Opin Investig Drugs 4: 1436–1441
Beeram M et al. (2005) Raf: a strategic target for therapeutic development against cancer. J Clin Oncol 23: 6771–6790
Wilhelm SM et al. (2004) BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64: 7099–7109
Wright JJ et al. (2005) Clinical trials referral resource. Current clinical trials of BAY 43-9006, Part 1. Oncology (Williston Park) 19: 499–502
Braga-Basaria M et al. (2004) 17-Allylamino-17-demethoxygeldanamycin activity against thyroid cancer cell lines correlates with heat shock protein 90 levels. J Clin Endocrinol Metab 89: 2982–2988
Schoenberger J et al. (2004) Effects of PTK787/ZK222584, a tyrosine kinase inhibitor, on the growth of a poorly differentiated thyroid carcinoma: an animal study. Endocrinology 145: 1031–1038
Younes MN et al. (2005) Antivascular therapy of human follicular thyroid cancer experimental bone metastasis by blockade of epidermal growth factor receptor and vascular growth factor receptor phosphorylation. Cancer Res 65: 4716–4727
Frantz S (2005) Drug discovery: playing dirty. Nature 437: 942–943
Weeraratna AT et al. (2001) Pan-trk inhibition decreases metastasis and enhances host survival in experimental models as a result of its selective induction of apoptosis of prostate cancer cells. Clin Cancer Res 7: 2237–2245
Mineo R et al. (2004) Activation of the hepatocyte growth factor (HGF)-Met system in papillary thyroid cancer: biological effects of HGF in thyroid cancer cells depend on Met expression levels. Endocrinology 145: 4355–4365
Corso S et al. (2005) Cancer therapy: can the challenge be MET? Trends Mol Med 11: 284–292
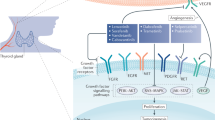
St Bernard R et al. (2005) Fibroblast growth factor receptors as molecular targets in thyroid carcinoma. Endocrinology 146: 1145–1153
Mitsiades CS et al. (2004) Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell 5: 221–230
Schiff BA et al. (2004) Epidermal growth factor receptor (EGFR) is overexpressed in anaplastic thyroid cancer, and the EGFR inhibitor gefitinib inhibits the growth of anaplastic thyroid cancer. Clin Cancer Res 10: 8594–8602
Nobuhara Y et al. (2005) Efficacy of epidermal growth factor receptor-targeted molecular therapy in anaplastic thyroid cancer cell lines. Br J Cancer 92: 1110–1116
Podtcheko A et al. (2003) The selective tyrosine kinase inhibitor, STI571, inhibits growth of anaplastic thyroid cancer cells. J Clin Endocrinol Metab 88: 1889–1896
Dziba JM and Ain KB (2004) Imatinib mesylate (Gleevec; STI571) monotherapy is ineffective in suppressing human anaplastic thyroid carcinoma cell growth in vitro. J Clin Endocrinol Metab 89: 2127–2135
Pietras K et al. (2002) Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res 62: 5476–5484
Kada F et al. (2004) Akt: a potential target for thyroid cancer therapy. Curr Drug Targets Immune Endocr Metabol Disord 4: 181–185
Wu G et al. (2005) Uncommon mutation but common amplifications of the PIK3CA gene in thyroid tumors. J Clin Endocrinol Metab 90: 4688–4693
Mandal M et al. (2005) The Akt inhibitor KP372-1 suppresses Akt activity and cell proliferation and induces apoptosis in thyroid cancer cells. Br J Cancer 92: 1899–1905
Bjornsti MA and Houghton PJ (2004) The TOR pathway: a target for cancer therapy. Nat Rev Cancer 4: 335–348
Visconti R et al. (1997) Expression of the neoplastic phenotype by human thyroid carcinoma cell lines requires NFκB p65 protein expression. Oncogene 15: 1987–1994
Pacifico F et al. (2004) Oncogenic and anti-apoptotic activity of NF-κB in human thyroid carcinomas. J Biol Chem 279: 54610–54619
Karin M et al. (2004) The IKK NF-κB system: a treasure trove for drug development. Nat Rev Drug Discov 3: 17–26
Nikiforov YE (2005) Anaplastic carcinoma of the thyroid—will aurora B light a path for treatment? J Clin Endocrinol Metab 90: 1243–1245
Sorrentino R et al. (2005) Aurora B overexpression associates with the thyroid carcinoma undifferentiated phenotype and is required for thyroid carcinoma cell proliferation. J Clin Endocrinol Metab 90: 928–935
Andrews PD (2005) Aurora kinases: shining lights on the therapeutic horizon? Oncogene 24: 5005–5015
McInnes C et al. (2005) Progress in the discovery of polo-like kinase inhibitors. Curr Top Med Chem 5: 181–197
Entrez Gene [http://www.ncbi.nlm.nih.gov/entrez/]
Catalogue of Somatic Mutations in Cancer [http://www.sanger.ac.uk/genetics/CGP/cosmic/]
Acknowledgements
We thank members of our laboratory for their comments and advice and Jean A Gilder for text editing. We apologize to the many colleagues whose work we have not cited because of space limits.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that their research has been partly supported by grants from AstraZeneca (Alderley Park, Macclesfield, Cheshire, UK) and Bayer HealthCare Pharmaceuticals (West Haven, Connecticut, USA).
Rights and permissions
About this article
Cite this article
Santoro, M., Carlomagno, F. Drug Insight: small-molecule inhibitors of protein kinases in the treatment of thyroid cancer. Nat Rev Endocrinol 2, 42–52 (2006). https://doi.org/10.1038/ncpendmet0073
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0073
This article is cited by
-
Vandetanib: opening a new treatment practice in advanced medullary thyroid carcinoma
Endocrine (2013)
-
A Phase I Study of the combination of oxaliplatin/docetaxel and vandetanib for the treatment of advanced gastroesophageal cancer
Investigational New Drugs (2013)
-
Partial remission of metastatic papillary thyroid carcinoma with sunitinib. Report of a case and review of the literature
Endocrine (2010)
-
New therapeutic approaches to treat medullary thyroid carcinoma
Nature Clinical Practice Endocrinology & Metabolism (2008)
-
Thyroid carcinoma: molecular pathways and therapeutic targets
Modern Pathology (2008)