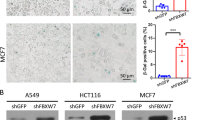
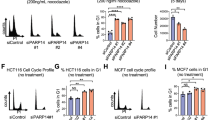
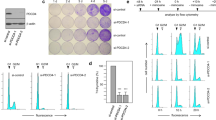
Abstract
p53 limits the proliferation of primary diploid fibroblasts by inducing a state of growth arrest named replicative senescence — a process which protects against oncogenic transformation and requires integrity of the p53 tumour suppressor pathway1,2,3. However, little is known about the downstream target genes of p53 in this growth-limiting response. Here, we report that suppression of the p53 target gene encoding plasminogen activator inhibitor-1 (PAI-1) by RNA interference (RNAi) leads to escape from replicative senescence both in primary mouse embryo fibroblasts and primary human BJ fibroblasts. PAI-1 knockdown results in sustained activation of the PI(3)K–PKB–GSK3β pathway and nuclear retention of cyclin D1, consistent with a role for PAI-1 in regulating growth factor signalling. In agreement with this, we find that the PI(3)K–PKB–GSK3β–cyclin D1 pathway is also causally involved in cellular senescence. Conversely, ectopic expression of PAI-1 in proliferating p53-deficient murine or human fibroblasts induces a phenotype displaying all the hallmarks of replicative senescence. Our data indicate that PAI-1 is not merely a marker of senescence, but is both necessary and sufficient for the induction of replicative senescence downstream of p53.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lundberg, A. S., Hahn, W. C., Gupta, P. & Weinberg, R. A. Genes involved in senescence and immortalization. Curr. Opin. Cell Biol. 12, 705–709 (2000).
Sherr, C. J. & McCormick, F. The RB and p53 pathways in cancer. Cancer Cell 2, 103–112 (2002).
Massague, J. G1 cell-cycle control and cancer. Nature 432, 298–306 (2004).
Pantoja, C. & Serrano, M. Murine fibroblasts lacking p21 undergo senescence and are resistant to transformation by oncogenic Ras. Oncogene 18, 4974–4982 (1999).
Kunz, C., Pebler, S., Otte, J. & von der Ahe, D. Differential regulation of plasminogen activator and inhibitor gene transcription by the tumor suppressor p53. Nucleic Acids Res. 23, 3710–3717 (1995).
Zhao, R. et al. Analysis of p53-regulated gene expression patterns using oligonucleotide arrays. Genes Dev. 14, 981–993 (2000).
Mu, X. C. & Higgins, P. J. Differential growth state-dependent regulation of plasminogen activator inhibitor type-1 expression in senescent IMR-90 human diploid fibroblasts. J. Cell Physiol. 165, 647–657 (1995).
Martens, J. W. et al. Aging of stromal-derived human breast fibroblasts might contribute to breast cancer progression. Thromb. Haemost. 89, 393–404 (2003).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
De Petro, G., Copeta, A. & Barlati, S. Urokinase-type and tissue-type plasminogen activators as growth factors of human fibroblasts. Exp. Cell Res. 213, 286–294 (1994).
Brummelkamp, T. R., Bernards, R. & Agami, R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell 2, 243–247 (2002).
Andreasen, P. A., Egelund, R. & Petersen, H. H. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol. Life Sci. 57, 25–40 (2000).
Choong, P. F. & Nadesapillai, A. P. Urokinase plasminogen activator system: a multifunctional role in tumor progression and metastasis. Clin. Orthop. 415, S46–S58 (2003).
Quelle, D. E. et al. Cloning and characterization of murine p16INK4a and p15INK4b genes. Oncogene 11, 635–645 (1995).
Linardopoulos, S. et al. Deletion and altered regulation of p16INK4a and p15INK4b in undifferentiated mouse skin tumors. Cancer Res. 55, 5168–5172 (1995).
Sherr, C. J. Tumor surveillance via the ARF-p53 pathway. Genes Dev. 12, 2984–2991 (1998).
Cross, D. A., Alessi, D. R., Cohen, P., Andjelkovich, M. & Hemmings, B. A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 (1995).
Vivanco, I. & Sawyers, C. L. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nature Rev. Cancer 2, 489–501 (2002).
Diehl, J. A., Cheng, M., Roussel, M. F. & Sherr, C. J. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 12, 3499–3511 (1998).
Chandrasekar, N. et al. Downregulation of uPA inhibits migration and PI3k/Akt signaling in glioblastoma cells. Oncogene 22, 392–400 (2003).
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995).
Parsons, R. Human cancer, PTEN and the PI-3 kinase pathway. Semin. Cell Dev. Biol. 15, 171–176 (2004).
Radu, A., Neubauer, V., Akagi, T., Hanafusa, H. & Georgescu, M. M. PTEN induces cell cycle arrest by decreasing the level and nuclear localization of cyclin D1. Mol. Cell Biol. 23, 6139–6149 (2003).
Dannenberg, J. H., van Rossum, A., Schuijff, L. & te Riele, H. Ablation of the retinoblastoma gene family deregulates G(1) control causing immortalization and increased cell turnover under growth-restricting conditions. Genes Dev. 14, 3051–3064 (2000).
Sage, J. et al. Targeted disruption of the three Rb-related genes leads to loss of G(1) control and immortalization. Genes Dev. 14, 3037–3050 (2000).
Rowland, B. D. et al. E2F transcriptional repressor complexes are critical downstream targets of p19(ARF)/p53-induced proliferative arrest. Cancer Cell 2, 55–65 (2002).
West, M. D., Shay, J. W., Wright, W. E. & Linskens, M. H. Altered expression of plasminogen activator and plasminogen activator inhibitor during cellular senescence. Exp. Gerontol. 31, 175–193 (1996).
Brown, J. P., Wei, W. & Sedivy, J. M. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science 277, 831–834 (1997).
Berns, K. et al. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature 428, 431–437 (2004).
Chen, Z. et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436, 725–730 (2005).
Tuveson, D. A. et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 5, 375–387 (2004).
Prendergast, G. C., Diamond, L. E., Dahl, D. & Cole, M. D. The c-myc-regulated gene mrl encodes plasminogen activator inhibitor 1. Mol. Cell Biol. 10, 1265–1269 (1990).
Parfyonova, Y. V., Plekhanova, O. S. & Tkachuk, V. A. Plasminogen activators in vascular remodeling and angiogenesis. Biochemistry (Mosc) 67, 119–134 (2002).
Bissell, M. J. et al. Tissue structure, nuclear organization and gene expression in normal and malignant breast. Cancer Res. 59, S1757–S1763 (1999).
Usher, P. A. et al. Expression of urokinase plasminogen activator, its receptor and type-1 inhibitor in malignant and benign prostate tissue. Int. J. Cancer 113, 870–880 (2005).
Frandsen, T. L. et al. Direct evidence of the importance of stromal urokinase plasminogen activator (uPA) in the growth of an experimental human breast cancer using a combined uPA gene-disrupted and immunodeficient xenograft model. Cancer Res. 61, 532–537 (2001).
Almholt, K. et al. Reduced metastasis of transgenic mammary cancer in urokinase-deficient mice. Int. J. Cancer 113, 525–532 (2005).
Tuxhorn, J. A., Ayala, G. E. & Rowley, D. R. Reactive stroma in prostate cancer progression. J. Urol. 166, 2472–2483 (2001).
Mueller, M. M. & Fusenig, N. E. Friends or foes — bipolar effects of the tumour stroma in cancer. Nature Rev. Cancer 4, 839–849 (2004).
Dirac, A. M. & Bernards, R. Reversal of senescence in mouse fibroblasts through lentiviral suppression of p53. J. Biol. Chem. 278, 11731–11734 (2003).
Yu, J. Y., Taylor, J., DeRuiter, S. L., Vojtek, A. B. & Turner, D. L. Simultaneous inhibition of GSK3α and GSK3β using hairpin siRNA expression vectors. Mol. Ther. 7, 228–236 (2003).
Acknowledgements
We would like to thank A. Visser for technical assistance, K. Berns, M. Hijmans, A. Dirac, T. Brummelkamp, R. Agami, R. van der Kammen, J. Collard and F. Scheeren for retroviral constructs, B. Weigelt for help with QRT–PCR, F. Foijer for retinoblastoma family deficient MEFs, L. Oomen and L. Brocks for help with microscopy, and R. Beijersbergen and D. Peeper for helpful discussions. This work was supported by a grant from the Dutch Cancer Society to R.B. and a grant from the National Institutes of Health (NIH; GM57242) to P.H.
Author information
Authors and Affiliations
Contributions
R.K. and R.B. conceived and designed the experiments. R.K. performed the experiments. P.H. contributed materials. R.K. and R.B. analysed the data. R.K. and R.B. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4 and S5 (PDF 9967 kb)
Rights and permissions
About this article
Cite this article
Kortlever, R., Higgins, P. & Bernards, R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol 8, 877–884 (2006). https://doi.org/10.1038/ncb1448
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1448
This article is cited by
-
KDM4C-mediated senescence defense is a targetable vulnerability in gastric cancer harboring TP53 mutations
Clinical Epigenetics (2023)
-
Serpin E1 mediates the induction of renal tubular degeneration and premature senescence upon diabetic insult
Scientific Reports (2023)
-
SIRT6-PAI-1 axis is a promising therapeutic target in aging-related bone metabolic disruption
Scientific Reports (2023)
-
Dual SMAD inhibition enhances the longevity of human epididymis epithelial cells
Cell and Tissue Research (2023)
-
Cellular Aging Secretes: a Comparison of Bone-Marrow-Derived and Induced Mesenchymal Stem Cells and Their Secretome Over Long-Term Culture
Stem Cell Reviews and Reports (2023)