Abstract
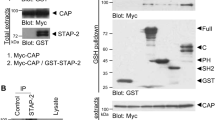
Insulin stimulates the transport of glucose into fat and muscle cells. Although the precise molecular mechanisms involved in this process remain uncertain, insulin initiates its actions by binding to its tyrosine kinase receptor, leading to the phosphorylation of intracellular substrates. One such substrate is the Cbl protooncogene product1. Cbl is recruited to the insulin receptor by interaction with the adapter protein CAP, through one of three adjacent SH3 domains in the carboxy terminus of CAP2. Upon phosphorylation of Cbl, the CAP–Cbl complex dissociates from the insulin receptor and moves to a caveolin-enriched, triton-insoluble membrane fraction3. Here, to identify a molecular mechanism underlying this subcellular redistribution, we screened a yeast two-hybrid library using the amino-terminal region of CAP and identified the caveolar protein flotillin. Flotillin forms a ternary complex with CAP and Cbl, directing the localization of the CAP–Cbl complex to a lipid raft subdomain of the plasma membrane. Expression of the N-terminal domain of CAP in 3T3-L1 adipocytes blocks the stimulation of glucose transport by insulin, without affecting signalling events that depend on phosphatidylinositol-3-OH kinase. Thus, localization of the Cbl–CAP complex to lipid rafts generates a pathway that is crucial in the regulation of glucose uptake.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ribon, V. & Saltiel, A. R. Insulin stimulates tyrosine phosphorylation of the proto-oncogene product of c-Cbl in 3T3-L1 adipocytes. Biochem. J. 324, 839–845 ( 1997).
Ribon, V., Printen, J. A., Hoffman, N. G., Kay, B. K. & Saltiel, A. R. A novel, multifuntional c-Cbl binding protein in insulin receptor signaling in 3T3-L1 adipocytes. Mol. Cell. Biol. 18, 872–879 (1998).
Mastick, C. C., Brady, M. J. & Saltiel, A. R. Insulin stimulates the tyrosine phosphorylation of caveolin. J. Cell Biol. 129, 1523– 1531 (1995).
Printen, J. A., Brady, M. J. & Saltiel, A. R. PTG, a protein phosphatase 1-binding protein with a role in glycogen metabolism. Science 275, 1475–1478 (1997).
Bickel, P. E. et al. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J. Biol. Chem. 272, 13793–13802 ( 1997).
Anderson, R. G. The caveolae membrane system. Annu. Rev. Biochem. 67 , 199–225 (1998).
Harder, T. & Simons, K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr. Opin. Cell. Biol. 9, 534–542 ( 1997).
Volonte, D. et al. Flotillins/cavatellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo. Characterization and epitope-mapping of a novel flotillin-1 monoclonal antibody probe. J. Biol. Chem. 274, 12702–12709 (1999).
Thurmond, D. C. et al. Regulation of insulin-stimulated GLUT4 translocation by Munc18c in 3T3L1 adipocytes. J. Biol. Chem. 273, 33876–33883 (1998).
Gustavsson, J. et al. Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB J. 13, 1961– 1971 (1999).
Mastick, C. C. & Saltiel, A. R. Insulin-stimulated tyrosine phosphorylation of caveolin is specific for the differentiated adipocyte phenotype in 3T3-L1 cells. J. Biol. Chem. 272, 20706–20714 (1997).
Corvera, S. & Czech, M. P. Direct targets of phosphoinositide 3-kinase products in membrane traffic and signal transduction. Trends Cell Biol. 8, 442–446 (1998).
Keller, S. R., Scott, H. M., Mastick, C. C., Aebersold, R. & Lienhard, G. E. Cloning and characterization of a novel insulin-regulated membrane aminopeptidase from Glut4 vesicles. J. Biol. Chem. 270, 23612– 23618 (1995); erratum, ibid 270, 30236 (1995)..
Kandror, K. V. & Pilch, P. F. gp160, a tissue-specific marker for insulin-activated glucose transport. Proc. Natl Acad. Sci. USA 91, 8017–8021 ( 1994).
Oka, Y., Mottola, C., Oppenheimer, C. L. & Czech, M. P. Insulin activates the appearance of insulin-like growth factor II receptors on the adipocyte cell surface. Proc. Natl Acad. Sci. USA 81, 4028–4032 (1984).
Wardzala, L. J., Simpson, I. A., Rechler, M. M. & Cushman, S. W. Potential mechanism of the stimulatory action of insulin on insulin- like growth factor II binding to the isolated rat adipose cell. Apparent redistribution of receptors cycling between a large intracellular pool and the plasma membrane. J. Biol. Chem. 259, 8378– 8383 (1984).
Malide, D. & Cushman, S. W. Morphological effects of wortmannin on the endosomal system and GLUT4-containing compartments in rat adipose cells. J. Cell Sci. 110, 2795– 2806 (1997).
Min, J. et al. Synip: a novel insulin-regulated syntaxin 4-binding protein mediating GLUT4 translocation in adipocytes. Mol. Cell 3, 751–760 (1999).
Pessin, J. E., Thurmond, D. C., Elmendorf, J. S., Coker, K. J. & Okada, S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! Location! Location! J. Biol. Chem. 274, 2593–2596 ( 1999).
Czech, M. P. & Corvera, S. Signaling mechanisms that regulate glucose transport. J. Biol. Chem. 274, 1865 –1868 (1999).
Wiese, R. J., Mastick, C. C., Lazar, D. F. & Saltiel, A. R. Activation of mitogen-activated protein kinase and phosphatidylinositol 3′-kinase is not sufficient for the hormonal stimulation of glucose uptake, lipogenesis, or glycogen synthesis in 3T3-L1 adipocytes. J. Biol. Chem. 270, 3442–3446 (1995).
Isakoff, S. J. et al. The inability of phosphatidylinositol 3-kinase activation to stimulate GLUT4 translocation indicates additional signaling pathways are required for insulin-stimulated glucose uptake. Proc. Natl Acad. Sci. USA 92, 10247–10251 (1995).
Guilherme, A. & Czech, M. P. Stimulation of IRS-1-associated phosphatidylinositol 3-kinase and Akt/protein kinase B but not glucose transport by beta1-integrin signaling in rat adipocytes. J. Biol. Chem. 273, 33119–33122 (1998).
Jiang, T. et al. Membrane-permeant esters of phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273, 11017– 11024 (1998).
Ribon, V., Johnson, J. H., Camp, H. S. & Saltiel, A. R. Thiazolidinediones and insulin resistance: peroxisome proliferatoractivated receptor gamma activation stimulates expression of the CAP gene. Proc. Natl Acad. Sci. USA 95, 14751– 14756 (1998).
Kandror, K. V., Stephens, J. M. & Pilch, P. F. Expression and compartmentalization of caveolin in adipose cells: coordinate regulation with and structural segregation from GLUT4. J. Cell Biol. 129, 999– 1006 (1995).
Scherer, P. E. et al. Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J. Cell Biol. 127, 1233–1243 (1994).
Gustavsson, J., Parpal, S. & Stralfors, P. Insulin-stimulated glucose uptake involves the transition of glucose transporters to a caveolae-rich fraction within the plasma membrane: implications for type II diabetes. Mol. Med. 2, 367–372 (1996).
Lazar, D. F. et al. Mitogen-activated protein kinase kinase inhibition does not block the stimulation of glucose utilization by insulin. J. Biol. Chem. 270, 20801–20807 ( 1995).
Elmendorf, J. S., Chen, D. & Pessin, J. E. Guanosine 5′-O-(3-thiotriphosphate) (GTPγS) stimulation of GLUT4 translocation is tyrosine kinase-dependent. J. Biol. Chem. 273, 13289–13296 (1998).
Acknowledgements
J.E.P. is supported by the NIH. P.E.B. is supported by the American Diabetes Association and awards from the Washington University Clinical Nutrition Research Unit and Diabetes Research Training Center.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Baumann, C., Ribon, V., Kanzaki, M. et al. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 407, 202–207 (2000). https://doi.org/10.1038/35025089
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35025089
This article is cited by
-
Tirzepatide prevents neurodegeneration through multiple molecular pathways
Journal of Translational Medicine (2024)
-
KSRP improves pancreatic beta cell function and survival
Scientific Reports (2024)
-
Phosphoproteomics reveals rewiring of the insulin signaling network and multi-nodal defects in insulin resistance
Nature Communications (2023)
-
FLOT1 knockdown inhibits growth of AML cells through triggering apoptosis and pyroptosis
Annals of Hematology (2023)
-
Complementary omics strategies to dissect p53 signaling networks under nutrient stress
Cellular and Molecular Life Sciences (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.