Abstract
The selective estrogen receptor modulator tamoxifen is routinely used for treatment and prevention of estrogen-receptor-positive breast cancer. Studies of tamoxifen adherence suggest that over half of patients discontinue treatment before the recommended 5 years. We hypothesized that polymorphisms in CYP2D6, the enzyme responsible for tamoxifen activation, predict for tamoxifen discontinuation. Tamoxifen-treated women (n=297) were genotyped for CYP2D6 variants and assigned a ‘score’ based on predicted allele activities from 0 (no activity) to 2 (high activity). Correlation between CYP2D6 score and discontinuation rates at 4 months was tested. We observed a strong nonlinear correlation between higher CYP2D6 score and increased rates of discontinuation (r2=0.935, P=0.018). These data suggest that presence of active CYP2D6 alleles may predict for higher likelihood of tamoxifen discontinuation. Therefore, patients who may be most likely to benefit from tamoxifen may paradoxically be most likely to discontinue treatment prematurely.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Partridge AH, Avorn J, Wang PS, Winer EP . Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst 2002; 94: 652–661.
Fisher B, Dignam J, Bryant J, Wolmark N . Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst 2001; 93: 684–690.
Stewart HJ, Prescott RJ, Forrest AP . Scottish adjuvant tamoxifen trial: a randomized study updated to 15 years. J Natl Cancer Inst 2001; 93: 456–462.
Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 365: 1687–1717.
Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 2004; 350: 1081–1092.
Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol 2007; 25: 486–492.
Demissie S, Silliman RA, Lash TL . Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol 2001; 19: 322–328.
Maurice A, Howell A, Evans DG, O'Neil AC, Scobie S . Predicting compliance in a breast cancer prevention trial. Breast J 2006; 12: 446–450.
Powles T, Eeles R, Ashley S, Easton D, Chang J, Dowsett M et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet 1998; 352: 98–101.
Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA . Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 2006; 99: 215–220.
Partridge AH . Non-adherence to endocrine therapy for breast cancer. Ann Oncol 2006; 17: 183–184.
Chlebowski RT, Geller ML . Adherence to endocrine therapy for breast cancer. Oncology 2006; 71: 1–9.
Grunfeld EA, Hunter MS, Sikka P, Mittal S . Adherence beliefs among breast cancer patients taking tamoxifen. Patient Educ Couns 2005; 59: 97–102.
Atkins L, Fallowfield L . Intentional and non-intentional non-adherence to medication amongst breast cancer patients. Eur J Cancer 2006; 42: 2271–2276.
Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, snis-Alibozek A . Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 2008; 26: 556–562.
Osborne CK . Tamoxifen in the treatment of breast cancer. N Engl J Med 1998; 339: 1609–1618.
Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 2003; 95: 1758–1764.
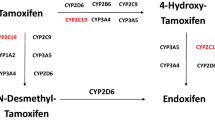
Desta Z, Ward BA, Soukhova NV, Flockhart DA . Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther 2004; 310: 1062–1075.
Jin Y, Desta Z, Stearns V, Ward B, Ho H, Lee KH et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 2005; 97: 30–39.
Borges S, Desta Z, Li L, Skaar TC, Ward BA, Nguyen A et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther 2006; 80: 61–74.
Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat 2004; 85: 151–159.
Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol 2005; 23: 9312–9318.
Cuzick J, Sestak I, Cella D, Fallowfield L . Treatment-emergent endocrine symptoms and the risk of breast cancer recurrence: a retrospective analysis of the ATAC trial. Lancet Oncol 2008; 9: 1143–1148.
Mortimer JE, Flatt SW, Parker BA, Gold EB, Wasserman L, Natarajan L et al. Tamoxifen, hot flashes and recurrence in breast cancer. Breast Cancer Res Treat 2008; 108: 421–426.
Bradford LD . CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 2002; 3: 229–243.
Blake MJ, Gaedigk A, Pearce RE, Bomgaars LR, Christensen ML, Stowe C et al. Ontogeny of dextromethorphan O- and N-demethylation in the first year of life. Clin Pharmacol Ther 2007; 81: 510–516.
Jin Y, Hayes DF, Li L, Robarge J, Skaar T, Phillips S et al. Estrogen receptor genotypes influence hot flash prevalence and composite score before and after tamoxifen therapy. J Clin Oncol 2008; 26: 5849–5854.
Goetz MP, Knox SK, Suman VJ, Rae JM, Safgren SL, Ames MM et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat 2007; 101: 113–121.
Bonanni B, Macis D, Maisonneuve P, Johansson HA, Gucciardo G, Oliviero P et al. Polymorphism in the CYP2D6 tamoxifen-metabolizing gene influences clinical effect but not hot flashes: data from the Italian Tamoxifen Trial. J Clin Oncol 2006; 24: 3708–3709.
Schroth W, Antoniadou L, Fritz P, Schwab M, Muerdter T, Zanger UM et al. Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J Clin Oncol 2007; 25: 5187–5193.
Wegman P, Vainikka L, Stal O, Nordenskjold B, Skoog L, Rutqvist LE et al. Genotype of metabolic enzymes and the benefit of tamoxifen in postmenopausal breast cancer patients. Breast Cancer Res 2005; 7: R284–R290.
Wegman P, Elingarami S, Carstensen J, Stal O, Nordenskjold B, Wingren S . Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer. Breast Cancer Res 2007; 9: R7.
Nowell SA, Ahn J, Rae JM, Scheys JO, Trovato A, Sweeney C et al. Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res Treat 2005; 91: 249–258.
Okishiro M, Taguchi T, Jin KS, Shimazu K, Tamaki Y, Noguchi S . Genetic polymorphisms of CYP2D6*10 and CYP2C19*2,*3 are not associated with prognosis, endometrial thickness, or bone mineral density in Japanese breast cancer patients treated with adjuvant tamoxifen. Cancer 2009; 115: 952–961.
Newman WG, Hadfield KD, Latif A, Roberts SA, Shenton A, McHague C et al. Impaired tamoxifen metabolism reduces survival in familial breast cancer patients. Clin Cancer Res 2008; 14: 5913–5918.
Ramon YC, Altes A, Pare L, Del RE, Alonso C, Barnadas A et al. Impact of CYP2D6 polymorphisms in tamoxifen adjuvant breast cancer treatment. Breast Cancer Res Treat 2009. [E-pub ahead of print].
Bijl MJ, van Schaik RH, Lammers LA, Hofman A, Vulto AG, van Gelder T et al. The CYP2D6*4 polymorphism affects breast cancer survival in tamoxifen users. Breast Cancer Res Treat 2009. [E-pub ahead of print].
Fellowes D, Fallowfield LJ, Saunders CM, Houghton J . Tolerability of hormone therapies for breast cancer: how informative are documented symptom profiles in medical notes for ‘well-tolerated’ treatments? Breast Cancer Res Treat 2001; 66: 73–81.
Osterberg L, Blaschke T . Adherence to medication. N Engl J Med 2005; 353: 487–497.
Horne R, Weinman J . Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 1999; 47: 555–567.
Partridge AH, Wang PS, Winer EP, Avorn J . Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol 2003; 21: 602–606.
Hayes DF . Clinical practice. Follow-up of patients with early breast cancer. N Engl J Med 2007; 356: 2505–2513.
Stearns V . Clinical update: new treatments for hot flushes. Lancet 2007; 369: 2062–2064.
Le Corre P, Parmer RJ, Kailasam MT, Kennedy BP, Skaar TP, Ho H et al. Human sympathetic activation by alpha2-adrenergic blockade with yohimbine: bimodal, epistatic influence of cytochrome P450-mediated drug metabolism. Clin Pharmacol Ther 2004; 76: 139–153.
Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS . The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther 2008; 83: 234–242.
Alfaro CL, Lam YW, Simpson J, Ereshefsky L . CYP2D6 inhibition by fluoxetine, paroxetine, sertraline, and venlafaxine in a crossover study: intraindividual variability and plasma concentration correlations. J Clin Pharmacol 2000; 40: 58–66.
Kotlyar M, Brauer LH, Tracy TS, Hatsukami DK, Harris J, Bronars CA et al. Inhibition of CYP2D6 activity by bupropion. J Clin Psychopharmacol 2005; 25: 226–229.
Skinner MH, Kuan HY, Pan A, Sathirakul K, Knadler MP, Gonzales CR et al. Duloxetine is both an inhibitor and a substrate of cytochrome P4502D6 in healthy volunteers. Clin Pharmacol Ther 2003; 73: 170–177.
Madeira M, Levine M, Chang TK, Mirfazaelian A, Bellward GD . The effect of cimetidine on dextromethorphan O-demethylase activity of human liver microsomes and recombinant CYP2D6. Drug Metab Dispos 2004; 32: 460–467.
Hamelin BA, Bouayad A, Methot J, Jobin J, Desgagnes P, Poirier P et al. Significant interaction between the nonprescription antihistamine diphenhydramine and the CYP2D6 substrate metoprolol in healthy men with high or low CYP2D6 activity. Clin Pharmacol Ther 2000; 67: 466–477.
Cella D, Fallowfield L, Barker P, Cuzick J, Locker G, Howell A . Quality of life of postmenopausal women in the ATAC (‘Arimidex’, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for early breast cancer. Breast Cancer Res Treat 2006; 100: 273–284.
Acknowledgements
We thank Dr C Kent Osborne and Dr Michael D Johnson for their valuable input. This work was supported in part by grants U-01 GM61373 and T-32 GM007767, Indiana University GCRC grant M01RR00750, University of Michigan GCRC grant M01-RR00042 and Georgetown University (NIH M01-RR13297), from the National Institute of General Medical Sciences (Bethesda, MD, USA), Damon Runyon-Lilly Clinical Investigator award CI-3 from the Damon Runyon Cancer Research Foundation (VS), Fashion Footwear Charitable Foundation of New York/QVC Presents Shoes on Sale (DFH) and Breast Cancer Research Foundation grant N003173 (JMR), and by grant number M01-RR000042 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Rae, J., Sikora, M., Henry, N. et al. Cytochrome P450 2D6 activity predicts discontinuation of tamoxifen therapy in breast cancer patients. Pharmacogenomics J 9, 258–264 (2009). https://doi.org/10.1038/tpj.2009.14
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2009.14
Keywords
This article is cited by
-
Adherence to endocrine therapy in early breast cancer in relation to Cytochrome P450 2D6 genotype: a comparison between pharmacy dispensation data and medical records
Breast Cancer Research and Treatment (2023)
-
Pharmacogenomics of breast cancer: highlighting CYP2D6 and tamoxifen
Journal of Cancer Research and Clinical Oncology (2020)
-
CYP2D6 genotype is not associated with survival in breast cancer patients treated with tamoxifen: results from a population-based study
Breast Cancer Research and Treatment (2017)
-
Persistence and discontinuation of adjuvant endocrine therapy in women with breast cancer
Breast Cancer (2016)
-
Limited predictive value of achieving beneficial plasma (Z)-endoxifen threshold level by CYP2D6 genotyping in tamoxifen-treated Polish women with breast cancer
BMC Cancer (2015)