Abstract
Pumping protons across a membrane was a critical step at the origin of life on earth and it is still performed in all living organisms, including in human cells. Proton pumping is paramount to keep normal cells alive, e.g. for lysosomal digestion and for preparing peptides for immune recognition, but it goes awry in cancer cells. They acidify their microenvironment hence membrane voltage is lowered, which in turn induces cell proliferation, a hallmark of cancer. Proton pumping is achieved by means of rotary motors, namely vacuolar ATPases (V-ATPase), which are present at many of the multiple cellular interfaces. Therefore, we undertook an examination of the thermodynamic properties of V-ATPases. The principal result is that the V-ATPase-mediated control of the cell membrane potential and the related and consequent environmental pH can potentially represent a valuable support strategy for anticancer therapies. A constructal theory approach is used as a new viewpoint to study how V-ATPase can be modulated for therapeutic purposes. In particular, V-ATPase can be regulated by using external fields, such as electromagnetic fields and a theoretical approach has been introduced to quantify the appropriate field strength and frequency for this new adjuvant therapeutic strategy.
Similar content being viewed by others
Introduction
A fundamental characteristic of intracellular membrane compartments is the difference between their luminal pH and the bulk cytoplasm pH. The vacuolar ATPase is the main mechanism responsible for this pH differential1. These proteins include a class of proton pumps structurally homologous to the F-ATPases that produce ATP by using the proton-motive force across the mitochondrial inner membrane2,3,4.
A vast amount of new information has been obtained on the structure, mechanics and biochemistry of the F-ATPases. For example, the rotation of the F1 motor was proven to develop over 40 pN nm and advance in three steps per revolution, with the hydrolysis of one ATP for each step5. Moreover, the F0 motor was found to consist of 10 or 12 subunits with rotational symmetry6. The F0 motor counters the large F1 torque by generating an even larger torque in the opposite direction to synthesize ATP. To do so it converts the transmembrane proton-motive force into rotary motion.
A mechanochemical model for the V-ATPase was suggested by Grabe, Wang and Oster2. This model allows us to predict proton pumping rates over a wide range of environmental conditions which proves useful to determine the acidification of organelles. The model is based on the hypothesis that the V-ATPase works under normal operating conditions and that ATP concentrations are sufficiently high so that hydrolysis is not rate limiting2. The V-ATPase structure is composed of a counter-rotating stator and a rotor. The membrane-inserted/transmembrane section V0 is affected by the hydrolysis of ATP in the V1-soluble headpiece. A two-channel model and a one-channel model have been suggested to explain the rotor-stator assemblies7. They differ in the protons' path through the enzyme and communicate with the cytoplasm through the protons bound to the rotor section2. However, some experiments on sodium V-ATPases seem to support the one channel model8.
The accepted model for the active transmembrane ion transport is the alternating access mechanism. Ions are bound tightly on the low concentration side of the membrane. A conformational change weakens their binding affinity by exposing them to the high concentration side; as a consequence, they dissociate. Then, the pump changes its conformation in order to begin the cycle again2.
These types of processes are usually described by mechanochemical approaches. But, recently, an applied thermodynamic approach has been developed to analyse the biophysical and biochemical behaviour of the biological systems9,10,11,12,13. This approach introduces a new method in biophysics and biochemistry: the differences stem from the conceptual bases of the approach itself11.
Cells are systems, as of yet too complex to fully identify and dissect the contribution of each component and the interactions among them. Consequently, it is difficult to model an ideal sequence inside the cell and to develop a well posed thermodynamic approach. As such, the cell is considered as a black box, which maintains communication with its environment. The subject of the approach is precisely this communication: it is characterized by heat and mass transfer between a well-known environment and an open system, the cell. So, our attention is focused on the spontaneous flows. In particular, cells discharge wasted heat. This heat is the consequence of the internal cellular biochemical reactions, which are irreversible processes. So, from an applied thermodynamic point of view the heat flows are the wasted heat for irreversible processes of an open system in a non-equilibrium state9,10,11. Consequently, the analysis of this irreversibility is fundamental to study the possible biological states of the cell: normal or diseased (e.g., cancerous). Indeed, normal and cancerous (or otherwise diseased) cells will dissipate different heat because different biochemical paths occur9,10. In applied thermodynamics, irreversibility is studied by introducing the concept of entropy generation and its link to the membrane flows12, as proven just by the constructal law12,13,14,15,16.
This paper will develop a constructal analysis of the V-ATPase and will suggest some bio-medical consequences, ultimately aimed at improving the presently availably anticancer therapies.
To do so, in Section 2 the thermodynamic approach will be summarised, while we develop in Section 3 its applicability for V-ATPase analysis and, in Section 4, we propose some biomedical hypotheses.
The approach to irreversibility
Cells are living systems. They grow and, at a proper time and depending on the tissues they belong to, each of them divides into two different daughter cells. At that time, the size of any cell can vary as well as their daughters' sizes, a phenomenon occurring within a particular range. So a cell's life is a cyclic process. Indeed, it begins with the emergence from cell separation and it ends with the separation of the daughter cells. Cells are composed of:
-
The cellular membrane, which controls the mass and energy inflow and outflow;
-
The cytoplasm, an aqueous solution containing thousands of structures and a vast array of chemical species;
-
The organelles, specialised subunits suspended in the cytoplasm, each enclosed within a membrane separating it from the cytoplasm. They perform specific, specialized, functions;
-
A network of tubular structures maintaining cell form and allowing directional transport such as microtubules, cilia, organizer.
Within cells, chemical reactions occur that produce energy and macromolecules and that increase and modify cell volume and its form17.
From a thermodynamic point of view, cells are open and complex systems able to convert their energy in the most efficient way for transport of substances across their membranes. They behave in two distinctively different ways: evolving towards maximum disorder or maintaining a high degree of organization in space and time. To do so, they must couple metabolic and chemical reactions with transport processes across their borders18.
Any system in nature has shape and structure13. They are macroscopic, finite size and recognizable as patterns. The previous classical thermodynamic analysis highlighted that the flows in cell systems are fundamental to evaluate the behaviour of the systems themselves. Consequently, the analysis of the flows through the cell membrane appears fundamental in the comprehension of the biophysical and biochemical mechanisms inside the cell12. But this kind of analysis is powerfully described by the constructal theory. Indeed, by referring to the constructal law, a living system presents two characteristics: it flows and it morphs freely toward configurations that allow all its currents to flow more easily over time13. Life and evolution are a physics phenomenon and they belong in physics16. Constructal law is a new approach introduced in thermodynamics in order to explain optimal shapes of natural structures13,14,15,16,19,20,21. The fundamental bases of the Constructal law was expressed21 as follows: “For a finite-size flow system to persist in time (to live), its configuration must evolve in such a way that provides greater and greater access to the currents that flow through it.”.
But, in a cell, a part of the energy is lost as heat outflow and only the resulting products of biochemical processes are known, while any individual step is inaccessible17. So, a constructal approach can represent a powerful theoretical method to analyse cell behaviour. Indeed, constructal theory highlights the fundamental role that flows across the system's border play in any thermodynamic process. This can represent a new viewpoint in the analysis of the biochemical and biophysical behaviour of cells. Instead of studying the cell, a very complex system, we can now study how the cells exchange components and information with their environments and the interactions between cells and environments, which consist of the flows across the cell membranes. Indeed, cells are so complex that it is very difficult to understand the single effect of a given cellular process in relation to the ‘global’ result for the cell. Consequently, the study of cells can be developed by introducing the black box model and considering only the spontaneous cell flows. Therefore the spontaneous heat cell exchange represents the interaction or, here, spontaneous communication between the cell and its environment. Lastly, it is, of course, easier to access the environment than the living cell.
Therefore, we decided to analyse the heat and mass flows across the membrane. This is what following a constructal approach suggests. But, in the analysis of the cell membrane, in relation to the molecular motors involved in this process, we have no useful data to evaluate directly the flows. Consequently we use constructal theory as a new fundamental viewpoint but we need a related method to develop the calculations. Notably, the heat flow is the consequence of the irreversible processes within cells and this is easily developed by using the Gouy-Stodola theorem22; it considers only the work lost for irreversibility and the temperature of the system's environment. This constructal based approach is theoretically interesting because22:
-
It allows to obtain the physical conditions in which an open system persists in its stationary states;
-
This is a power description of complex phenomena because it allows to evaluate the global effects and their fluctuations around the stationary states;
-
It involves the definition of exergy flows, which are the flows of the available energy of the system.
In 1873, Gibbs introduced the available energy, today named exergy. It is the function that expresses the maximum useful work that a system can obtain in a thermodynamic equilibrium with its environment. The exergy lost or dissipated Eλ, i.e. the available energy or work lost Wλ, in an irreversible process, for us the heat emerging from the cell, can be obtained through the Gouy-Stodola theorem21:

with T0 the environment temperature and Sg the entropy generation. By using this relation and evaluating any process across the membrane, always by using the well accepted applied thermodynamic relations21, the exergy flows across the membrane can be related to the entropy generation for a cell which has been recently obtained9,10,11,12 as:

where:
-
1
Sg,tf is the entropy generation due to the thermal flux driven by the temperature difference, in which τ1 is the lifetime of this process;
-
2
T is the temperature;
-
3
Sg,dc is the entropy generation due to the diffusion current driven by the chemical potential gradients, in which τ2 is the lifetime of this process, μi is the chemical potential of the i-th species;
-
4
Sg,vg is the entropy generation due to the velocity gradient coupled with the viscous stress, in which τ3 is the lifetime of this process;
-
5
Sg,cr is the entropy generation due to the chemical reaction rate driven by the affinity, in which τ4 is the lifetime of this process, N is the number per unit time and volume of the i-th chemical reaction and
 is the affinity, evaluated as the variation of the standard Gibbs' free energy;
is the affinity, evaluated as the variation of the standard Gibbs' free energy; -
6
Sg,de is the entropy generation due to the dissipation that results from the interaction between external forces and the system, in which τ5 is the lifetime of this process, F is the force generated by the interaction with the external field and J is the associated flow.
-
7
Where the volume of the cell is defined as12

with δ1(t) and δ2(t) the long and the short axes dimensions of the cell, but δ1(t) and δ2(t) must be experimentally evaluated, so for a theoretical approach the diameter of the cell is approximated as the diameter of a sphere12:

Consequently, the characteristic length of the cell results in:

-
8
r = L/2 is the cell radius
-
9
The mean environmental temperature can be assumed to be9,10,11,17T0 = 310 K and the mean cell temperature has been estimated to be T0 + ΔT;
-
10
ΔT is the difference between the temperature inside the cell and that of its environment9,10,11,12,17. It has been evaluated as ΔT ≈ 0.4°C, but it would be different for each cell line and for each cell line it would have to be different between normal and cancerous (or more generally, diseased) states9,10,11,12;
-
11
The characteristic length17,19,20 can be evaluated as L = 2 r;
-
12
The internal energy density u can be evaluated as the ratio between the cell's mean internal energy9,10,11,12,17,18, considered the same as that of ATP, U = 3 × 10−7 J and the mean value of the cell inside the human body V = 7600 μm3, hence the cell volume in the human body being in the range 200–15000 μm3, so it results in u = 3.95 × 107 Jm−3;
-
13
The thermal molecular mean velocity inside the cytoplasm is considered to be9,17
 ;
; -
14
de can be assessed as17de = 0.2 r;
-
15
The membrane volume is evaluated as9,10,11,12

with π = 3.14;
-
16
The chemical potential gradient9,10,11,12,17,18 can be calculated as the ratio between the mean value of the chemical potential μ = 1.20 × 10−9 J kg−1 and the membrane length dm = 0.01 μm, with the mean density being ρ = 1000 kg m−3;
-
17
The viscosity9,10,11,12,17 is taken to be 6.91 × 10−3 N s m−2;
- 18
- 19
-
20
Fk is the external field and Jk is the associated flow.
Constructal approach to V-ATPase
The basis of metabolism energetics consists in the generation and the hydrolysis of ATP, which occurs across a trans-membrane electromotive gradient. Indeed, this conversion of energy can be obtained by transitioning the electrochemical energy into the chemical energy of the terminal phosphoric anhydride bond of the ATP26. This can occur by the action of an enzyme, which works as a proton-pumping ATP synthetase. Here the proton pump V-ATPase will be discussed by introducing just the constructal approach.
As described in the introduction, the V-APTase works through a counter-rotating stator and a rotor mechanism. It hydrolyses ATP to obtain the required energy for its work. The fundamental reaction is:

and, consequently, a H+ ion is pumped into the cell:

where out means outside, in refers to inside and memb stands for across the membrane.
This proton-pumping can be modelled considering the membrane as an electric RC-circuit, while the V-ATPase can be modelled as a DC motor as represented in Figure 1. Indeed, the V-ATPase rotor can be considered to be the equivalent of a simple DC-motor rotor. The energy required by the rotor movement is generated by the energy conversion of the ATP-hydrolysis (7), while the stators rotate as gears dragged by the rotor itself. Moreover, all the DC motors convert electric energy into work with high efficiency (about 1), so, introducing this model for the rotor, the irreversibility results only in the gears. Consequently, the efficiency of the V-ATPase can be evaluated as2:

where ΔGATP is the free energy variation due to the hydrolysis of a single ATP molecule (~21 kBT = 50 kJ mol−1, being kB the Boltzmann constant and T the temperature), χ is the coupling ratio (χ = JH/JATP, being JH the proton flux and JATP the ATP hydrolysis rate) and ΔGP is the free energy variation required to move the proton across the membrane2:

with Δϕ being the membrane potential, R is the gas constant (8.314 J mol−1K−1), F is the Faraday constant (96.485 × 103 A s mol−1) and 2.3 ΔpΗ is the physiological concentration gradient. The coupling ratio χ is affected both by the pH gradients and by the membrane potential. The average rotation ν can be calculated as a function of the load τ as7:

Under physiological conditions, this leads23 to 15–20 Hz.
The equivalent schema for the V-ATPase system.
It is a DC motor in series with a rectifier. The V-ATPase rotor can be considered to be the equivalent of a simple DC-motor rotor. The energy required by the rotor movement is generated by the energy conversion of the ATP-hydrolysis, while the stators rotate as gears dragged by the rotor itself. The DC motor converts electric energy into work with high efficiency (about 1). Consequently, the irreversibility results only in the gears.
From these results the work dissipated in wasted heat yields:

and the related power lost in heat flow:

As a consequence of the previous Section it is now possible to evaluate the entropy generation due to membrane flows as:

and the related entropy generation rate as:

with Sg,tf being zero because the V-ATPase is not driven by temperature difference and Sg,vg being zero because the V-ATPase is not driven by the velocity gradient.
Considering relations (14) and (15) together with relations (12) and (13) it is now possible to argue that the entropy generation and consequently the irreversibility, depend on:
-
1
The chemical potential at the membrane,
-
2
The affinity,
-
3
The electric potential at the membrane,
-
4
The H+/ATP rate,
-
5
The pH gradient, and
-
6
The working temperature.
But, as highlighted in the introduction, these quantities are characteristic quantity of the thermodynamic state of a cell, which is different between normal and cancerous cells. Consequently, these quantities are also different between normal and cancerous cells of the same cell line. So, for a cancer cell it follows:


where the significant quantities are considered different from normal cells; to indicate it, a c symbol has been introduced. As a consequence, we get a different value of the entropy generation and the variation between a cancer cell and a normal cell leads to:


Furthermore, if we are able to change the entropic behaviour of the tumor cell it is possible to compel the cancer to behave as it would be a normal cell. To do so, the component Sg,de related to the dissipation due to work by interaction with the external field can be introduced obtaining the entropy generation (always multiplied with the environmental temperature to obtain energy balances as previously done) as:

and the related entropy generation rate:

To obtain the required effect it must be:


The effect could be obtained by using24,25,26,27,28,29,30,31:
-
1
catalysis32,
-
2
electric field interaction33,
-
3
electromagnetic or ultrasound waves,
-
4
molecular machines,
-
5
local inflow of nano-particles of ferro-fluids in interaction with a magnetic field, but this technique has yet to be designed,
and/or coupling some of these possible techniques.
Here, we want to highlight that this conceptual therapy is meant to become a supporting strategy to the currently applied anticancer therapies.
Considering the use of magnetic fields it is possible to argue that any such field would modify the rotation of the diseased cell towards the normal one. So, it must induce an electric field strong enough to obtain the normal rotation of the rotor. That means, the frequency of the electric field must be such that:

and it would supposedly to be in the 0 ÷ 40 Hz range, which is twice the physiological one and this will not damage the normal cell(s). The torque can be related to the membrane potential and results in values of the order of2 10−21 Nm. Now, this leads us to consider that this torque can be obtained also as34:

and that the relation between the electric field and a magnetic field for an electromagnetic wave is35:

with μm being the magnetic permeability and εe representing the electric permittivity of the cell membrane.
Considerations
All types of ATPases, i.e., the A-ATPase of Archaea, the E-,F-P- and the V-ATPases are essential for life and all of them generate an electrochemical ion gradient across the membrane and hydrolyse or synthesize ATP. Also, from a structural point of view, they are similar; indeed, they are enzymatic complexes, which work as molecular rotary motors. In particular, V-ATPase plays an important role36 in receptor-mediated endocytosis1, in intracellular transport and in the acidification of late endosomes37.
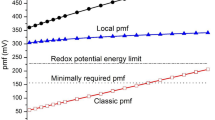
Metabolism is a cycle composed of a continuous sequence of oxidations and reductions. Considering our DC motor approach, the electromotive force is the free energy variation, ΔGP, required to move the proton across the membrane represented by the Nernst equation (10). From this equation it possible to highlight that any variation of the electromotive force of the V-ATPase rotor can determine two possible consequences:
-
1
A change in the membrane potential, or
-
2
A change in the pH.
Of course, these effects can also occur together.
V-ATPase supplies energy to the membrane, regulates intracellular pH and causes extracellular acidification or alkalinization. Its activity is fundamental for both vacuolar acidification in response to glucose metabolism and the regulation of pH homeostasis36. In particular, vacuolar acidification was found in the transport of lysosomal enzymes from the Golgi apparatus to the lysosomes38 and V-ATPase contributes to the homeostasis of cytoplasmic pH. Consequently, V-ATPase has a fundamental role in securing the microenvironment required for correct protein transport and membrane exchanges. As such, any breakdown in the V-ATPase can generate a different acid microenvironment. Indeed, V-ATPase breakdowns can cause lower pH in the microenvironment and this can be exploited by lysosomal enzymes of cancer cells that participate in the degradation of the extracellular matrix required for invasion and metastasis39,40.
Four regulatory mechanisms are known36:
-
1
The regulation of pump density, useful to maintain their cytoplasmic and vacuolar pH stable;
-
2
The regulation of V1 and V0 domain association/dissociation;
-
3
The regulation of secretory activity, thereby maintaining the balance in the formation of bisulfite and binding efficiency between H+ and the pump;
-
4
The modifications of the membrane potential due to electrogenic force.
Using a thermodynamic approach, the aim of this paper is to highlight how external electromagnetic waves can modify the mechanical behaviour of the V-ATPase rotor. So, now, we must evaluate the intensity and the frequency of the applied external magnetic field to obtain some beneficial anticancer effects yet without endangering the patient. To do so, we consider that any breakdown in the rotor can be modelled as a difference Δτ in the torque in comparison with the normal value τ, such that:

By using this last relation in equation (22) it follows that

with E + ΔE denoting the electric field related to the breakdown of the V-ATPase rotor. Consequently, the effect of an external magnetic field must be to contrast this field variation, so that, considering relation (26), it follows:

Now, considering a torque variation for the breakdown in the order of 0 ÷ 3 pN nm−1, so that the magnetic permittivity is about the value of the permittivity in the air, μm ~ 10−6 H m−1 and so that the electric permeability is around εe ~ 10−6 ÷ 10−5 F m−1,  ~ 10−12 ÷ 10−11 s m−1 and that the electric field at the membrane is around 107 V m−1 it follows that the therapeutic magnetic field must be of the order of 10−5 ÷ 10−4 T, i.e., of the same order as the Earth's magnetic field.
~ 10−12 ÷ 10−11 s m−1 and that the electric field at the membrane is around 107 V m−1 it follows that the therapeutic magnetic field must be of the order of 10−5 ÷ 10−4 T, i.e., of the same order as the Earth's magnetic field.
Now, from relation (24) we can obtain the possible range of frequencies of the magnetic wave. Considering that the normal value of the torque is around 10 pN nm−1, we assume a torque variation for the breakdown of the order of 0 ÷ 3 pN nm−1; so, it is possible to obtain a frequency range of the order 0 ÷ 10 kHz for therapeutic use.
Moreover, this frequency can be confirmed by evaluating the time τ2 related to the diffusion current driven by chemical potential gradients. It can be calculated by considering the macroscopic phenomenon of diffusion across the membrane as:

with d ≈ 10−8 m being the length of the membrane and D the diffusion coefficient. Considering that the diffusion coefficient of the ions across a membrane is around42 10−12 ÷ 10−8 m2s−1 it follows τ2 ≈ 10−4 ÷ 1 s, which is equivalent to a frequency ν = 1/τ2 of 0 ÷ 10 kHz.
Conclusions
Proteins play a fundamental role in ion transport because membranes represent a barrier to free diffusion of molecules.
In active transport an ion crosses the membrane against its electrochemical potential. The required energy for this process is obtained by the chemical energy released from hydrolysis of ATP, or pyrophosphate, or from the movement of a co-transported or coupled ion along its electrochemical gradient41. Indeed, the coupled downhill and uphill movement of ions is a common transport path through the membrane. In this context, the role of the pumps is fundamental, with particular regards to V-ATPase, i.e. the pump managing the H+ transport. Indeed, it moves positive charges into and from the cytoplasm through the hydrolysis of ATP, with the consequence of establishing a large membrane voltage (inside negative and outside positive) and, consequently, a pH gradient41 of about 400 mV for H+. The V-ATPase is composed of a membrane extrinsic and a membrane intrinsic sector and couples catalysis of ATP hydrolysis to proton transport by a rotational mechanism42. V-ATPase is fundamental in the analysis of cell behavior because the H+ gradient established by this molecular pump is used to drive coupled active movements of other ions across the cell membrane. One example is the Cl− transport: indeed, Cl− is actively transported across the membrane because the membrane potential is more negative than the equilibrium potential for this ion.
The ion channels and transporters provide different permeability to distinct ions, such as Na+, K+, Ca2+ and Cl−. As a consequence of the asymmetry in these ion distributions, a membrane potential exists between the cytoplasm and the extracellular environment. It is expressed relative to the extracellular environment and a cell depolarizes if the membrane potential is relatively less negative and vice versa43. The membrane potential can be calculated by using the Goldman–Hodgkin–Katz equation44,45:

where P is the permeability of the ion, [A] means concentration of the A-ion, R is the ideal gas constant (8.314 J mol−1K−1), T is the temperature and F is the Faraday constant (96.485 × 103 A s mol−1). From relation (31) it is possible to state that the membrane potential can be changed by alterations in the conductance of one or more ions. In particular, from the previous considerations on the V-ATPase we can highlight that this pump can change the transport of H+ and, as a consequence of the Cl−-H+ coupled transport, it can change the Cl− transport. Consequently, both the membrane potential and the pH are changed by any alteration of the V-ATPase.
It is noteworthy that in the analysis of the mitotic activities in sarcoma cells, the membrane potential was found to undergo hyper-polarization before entering M phase. It suggests that the level of Δϕ is correlated with cell cycle progression. Moreover, membrane hyper-polarization was shown to block reversibly DNA synthesis and mitosis and to be correlated with the level of differentiation46,47,48. Consequently, the membrane potential represents a fundamental quantity for the control of critical cell functions, particularly, with regards to proliferation, migration and differentiation. To support this conclusion, some experimental evidence can be cited: First, direct in vitro and in vivo comparisons of the membrane potentials have highlighted that cancer cells are more depolarized in relation to normal cells; some evidence of this electrochemical behavior of the cancer cells can be summarized as follows43: between normal and cancerous breast cells49, hepatocytes and hepatocellular carcinoma cells50, normal and neoplastic adrenocortical tissues51, normal embryonic fibroblasts and fibrosarcoma52, benign and cancerous skin cells53 and between normal and cancerous ovarian tissue54.
Lastly, cell migration is controlled by the movement of ions and water43 in that an acidic environment furthers this phenomenon. This environmental pH is regulated by the H+ concentration, which is related to the V-ATPase functions. In addition, the membrane potential is considered an indirect factor of cell migration, strictly related to the electrical driving force for Ca2+ whereas a hyperpolarized membrane potential increases intracellular Ca2+ through the TRP channels; in contrast, membrane depolarization activates the Ca2+ channels55. Notably, migrating cells have a high intracellular Ca2+ concentration gradient56.
In summary, all these findings highlight that V-ATPase-mediated control of the cell membrane potential and that of the environmental pH can potentially represent a valuable support strategy for anticancer therapies. In here, a constructal approach has been used to study how V-ATPase can be modulated for therapeutic purposes. In particular, V-ATPase can be regulated by using external fields, such as electromagnetic fields and we have introduced a theoretical approach to quantify the appropriate field strength and frequency for this new adjuvant therapeutic strategy. Here, in contrast with the usual applications of constructal theory57,58,59, the flows have been assessed by evaluating the entropy generation because its variation is strictly related to the flows60 across the cell membrane.
References
Forgac, M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Bio 8, 917–929 (2007).
Grabe, M., Wang, H. & Oster, G. The mechanochemistry of V-ATPase proton pumps. Biophs J. 78, 2798–2813 (2000).
Finbow, M. & Harrison, M. The vacuolar H+-ATPase: a universal proton pump of eukaryotes. Biochem. J. 324, 697–712 (1997).
Kibak, H., Taiz, L., Starke, T., Bernasconi, P. & Gogarten, J. P. Evolution of structure and function of V-ATPases. J. Bioenerg. Biomembr. 24, 415–424 (1992).
Noji, H., Yasuda, R., Yoshida, M. & Kinosita, K. Direct observation of the rotation of F1-ATPase. Nature 386, 299–302 (1997).
Nakamoto, R., Ketchum, C. & Al-Shawi, M. Rotational coupling in the F0F1 ATP synthase. Annu. Rev. Biophys. Biomol. Struct. 28, 205–234 (1999).
Elston, T., Wang, H. & Oster, G. Energy transduction in ATP synthase. Nature 391, 510–514 (1998).
Murata, T., Igarashi, K., Kakinuma, Y. & Yamato, I. Na+ binding of V-type Na+-ATPase in Enterococcus hirae. J. Biol. Chem. 275, 13415–13419 (2000).
Lucia, U. Entropy generation approach to cell systems. Physica A 406, 1–11 (2014).
Lucia, U. Entropy generation and cell growth with comments for a thermodynamic anticancer approach. Physica A 406, 107–118 (2014).
Lucia, U. Thermodynamic approach to nano-properties of cell membrane. Physica A 407, 185–191 (2014).
Lucia, U. Transport processes and irreversible thermodynamics analysis in tumoral systems. Physica A 410, 380–390 (2014).
Bejan, A. Shape and Structure, from Engineering to Nature (Cambridge: Cambridge University Press, 2000).
Bejan, A. & Lorente, S. The constructal law and the thermodynamics of flow systems with configuration. Int J Heat Mass Tran 47, 3203–3214 (2004).
Bejan, A. & Lorente, S. Constructal theory of generation of configuration in nature and engineering. J Appl Phys 100, 041301 (2006).
Bejan, A. & Lorente, S. Constructal law of design and evolution: Physics, biology, technology and society. J Appl Phys 113, 151301 (2013).
Mercer, W. B. 1971. The living cell as an open thermodynamic system: bacteria and irreversible thermodynamics. Technical Manuscript 640, May 1971, Approved for public release – distribution unlimited, U.S. Department of the Army, Fort Detrick, Frederick, Maryland, web page: www.dtic.mil/dtic/tr/fulltext/u2/726932.pdf (Last date of access: August, 08th, 2014).
Demirel, Y. & Sandler, S. I. Thermodynamics and bioenergetics. Biophys Chem 97, 87–111 (2002).
Bejan, A. Why the bigger live longer and travel farther: animals, vehicles, rivers and the winds. Sci. Rep. 2, 594 (2012).
Reis, A. H. Constructal theory: from engineering to physics and how flow systems develop shape and structure. Appl. Mech. Rev. 59, 269–281 (2006).
Bejan, A. Advanced Engineering Thermodynamics. (Hoboken: John Wiley, 2006).
Lucia, U. Stationary open systems: a brief review on contemporary theories on irreversibility. Physica A 392, 1051–1062 (2013).
Dimroth, P., Wang, H., Grabe, M. & Oster, G. Energy transduction in the sodium F-ATPase of Propionigenium modestum. PNAS. 96, 4924–4929 (1999).
Wiseman, H. M. & Eisert, J. Nontrivial quantum effects in biology: A skeptical physicists' view, Invited contribution to Abbott, D., Davies, P. T. W. & Pati, A. K. (Eds). Quantum Aspects of Life (London: Imperial College Press, 2008).
Engel, G. S. et al. Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature 446, 782–784 (2007).
Lee, H., Cheng, Y.-C. & Fleming, G. R. Coherence dynamics in photosynthesis: protein protection of excitonic Coherence. Science 316, 1462–1465 (2007).
Plenio, M. B. & Huelga, S. F. Dephasing assisted transport: quantum networks and biomolecules. New J Phys 10, 113019 (2008).
Hughes, A. L. Adaptive Evolution of Genes and Genomes (Oxford: Oxford University Press, 1999).
Browne, W. R. & Feringa, B. L. Making molecular machines work. Nat Nanotechnol 1, 25–35 (2006).
Alberts, B. et al. Molecular Biology of the Cell. 5th Edition (New York: Garland Science, Taylor & Francis, 2008).
Cai, J.-M., Popescu, S. & Briegel, H. J. Dynamical entanglement in oscillating molecules and potential biological implications. arXiv.,0809.4906 (2008).
Briegel, H. J. & Popescu, S. Intra-molecular refrigeration in enzymes. Proc. R. Soc. A 469, 20110290 (2013).
Liu, Y.-S. & Chen, Y.-C. Single-molecule refrigerators: substitution and gate effects. Appl Phys Lett 98, 213103 (2011).
Miller, J. H., Jr, Rajapakshe, K. I., Infante, H. L. & Claycomb, J. R. Electric Field Driven Torque in ATP Synthase. PLoS ONE 8, e74978 (2013).
Feynman, R. P., Leighton, R. B. & Sands, M. The Feynman Lectures on Physics. Vol. II. Part 2 (Boston: Addison-Wesley Publishing Company, 1963).
Pérez-Sayáns, M. et al. An update in the structure, function and regulation of V-ATPases: the role of the C subunit. Braz J Biol 72, 189-198 (2012).
Forgac, M. Structure, function and regulation of the vacuolar (H+)-ATPases. FEBS Lett 440, 258–263 (1998).
Moriyama, Y. & Nelson, N. H+-translocating ATPase in Golgi apparatus. Characterization as vacuolar H+-ATPase and its subunit structures. J Biol Chem 264, 18445–18450 (1989).
Martínez-Aaguilan, R., Lynch, R. M., Martinez, G. M. & Gillies, R. J. Vacuolar-type H(+)-ATPases are functionally expressed in plasma membranes of human tumor cells. Am J Physiol 265, C1015–1029 (1993).
Stevens, T. H. & Forgac, M. Structure, function and regulation of the vacuolar (H+)-ATPase. Annu Rev Cell Dev Bi 13, 779–808 (1997).
Tuszynski, J. A. & Kurzynski, M. Introduction to Molecular Biophysics (Boca Raton: CRC Press, 2003).
Nakanishi-Matsui, M., Sekiya, M., Nakamoto, R. K. & Futai, M. The mechanism of rotating proton pumping ATPases. BBA- Bioenergetics 1797, 1343–1352 (2010).
Yang, M. & Brackenbury, W. J. Membrane potential and cancer progression. Front Physiol 4, 185-1-10 (2013).
Goldman, D. E. Potential, impedance and rectification in membranes. J Gen Physiol 27, 37–60 (1943).
Hodgkin, A. L. & Katz, B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol 108, 37–77 (1949).
Cone, C. D. Jr. Electro-osmotic interactions accompanying mitosis initation in sarcoma cells in vitro. T New York Acad Sci 31, 404–427 (1969).
Cone, C. D., Jr Variation of the trans-membrane potential level as a basic mechanism of mitosis control. Oncology 24, 438–470 (1970).
Cone, C. D. Jr. Unified theory on the basic mechanism of normal mitotic control and oncogenesis. J Theor Biol 30, 151–181 (1971).
Marino, A. A., Morris, D. M., Schwalke, M. A., Iliev, I. G. & Rogers, S. Electrical potential measurements in human breast cancer and benign lesions. Tumor Biol 15, 147–152 (1994).
Stevenson, D. et al. Relationship between cell membrane potential and natural killer cell cytolysis in human hepatocellular carcinoma cells. Cancer Res 49, 4842–4845 (1989).
Lymangrover, J., Pearlmutter, A. F., Franco-Saenz, R. & Saffran, M. Transmembrane potentials and steroidogenesis in normal and neoplastic human adrenocortical tissue. J Clin Endocr Metab 41, 697–706 (1975).
Binggeli, R. & Weinstein, R. C. Deficits in elevating membrane potential of rat fibrosarcoma cells after cell contact. Cancer Res 45, 235–241 (1985).
Melczer, N. & Kiss, J. Electrical method for detection of early cancerous growth of the skin. Nature 179, 1177–1179 (1957).
Redmann, K., Muller, V., Tanneberger, S. & Kalkoff, W. The membrane potential of primary ovarian tumor cells in vitro and its dependence on the cell cycle. Acta Biol Med Ger 28, 853–856 (1972).
Schwab, A., Fabian, A., Hanley, P. J. & Stock, C. Role of ion channels and transporters in cell migration. Physioll Rev 92, 1865–1913 (2012).
Brundage, R. A., Fogarty, K. E., Tuft, R. A., & Fay, F. S. Calcium gradients underlying polarization and chemotaxis of eosinophils. Science 254, 703–706 (1991).
Silva, C. & Reis, A. H. Heart rate, arterial distensibility and optimal performance of the arterial tree. J Biomech 47, 2878–2882 (2014).
Reis, A. H., Miguel, A. F. & Aydin, M. Constructal theory of flow architecture of the lungs. Med. Phys. 31, 1135–1140 (2004).
Bejan, A., Ziaei, S. & Lorente, S. Evolution: Why all plumes and jets evolve to round cross sections. Sci Rep 4, 4730 (4730).
Lucia, U. The Gouy-Stodola Theorem in Bioenergetic Analysis of Living Systems (Irreversibility in Bioenergetics of Living Systems). Energies 7, 5717–5739 (2014).
Author information
Authors and Affiliations
Contributions
U.L. developed the thermodynamic approach. U.L., A.P. and T.S.D. contributed to the main manuscript text. All authors (U.L., A.P., T.S.D.) reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Lucia, U., Ponzetto, A. & Deisboeck, T. A thermo-physical analysis of the proton pump vacuolar-ATPase: the constructal approach. Sci Rep 4, 6763 (2014). https://doi.org/10.1038/srep06763
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06763
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.

 is the affinity, evaluated as the variation of the standard Gibbs' free energy;
is the affinity, evaluated as the variation of the standard Gibbs' free energy;


 ;
;
 is evaluated as
is evaluated as