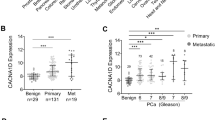
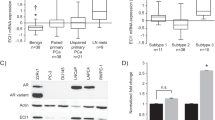
Abstract
The progression of prostate cancer from androgen-responsive to an androgen-unresponsive state remains the greatest obstacle in the treatment of this disease. Androgen-unresponsive prostate cancer is highly resistant to chemotherapy and radiation treatment that kill cells by the induction of apoptosis. Elucidating the molecular mechanisms of apoptosis regulation in prostate cancer can be useful in the development of new strategies for effective therapy of androgen-unresponsive cancer. We analyzed the Bcl-2 family of apoptosis regulators using various passages of the LNCaP prostate cancer cell line, which serve as an in vitro model for the progression of prostate cancer from androgen-responsive to androgen-unresponsive. In our model, progressively higher passages of LNCaP cells represent the progression to androgen-unresponsiveness. We examined the basal mRNA expression of the Bcl-2 family of apoptosis regulators. Under normal growth conditions, both androgen-responsive and androgen-unresponsive LNCaP cells express the Bcl-2 family of genes at similar levels. Western blot analysis showed the presence of Bcl-2 protein in androgen-responsive cells but not in androgen-unresponsive cells. Both androgen-responsive and androgen-unresponsive cells expressed Bax protein at similar levels. When exposed to oxidative stress, androgen-responsive cells underwent apoptosis but androgen-unresponsive cells exhibited resistance suggesting that the progression to androgen-unresponsiveness was associated with altered regulation of apoptosis. Treatment with paclitaxel or sodium butyrate induced apoptosis in both androgen-responsive and androgen-unresponsive cells suggesting that the apoptotic machinery is still intact in androgen-unresponsive LNCaP cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Boring CC, Squires TS, Tong T . Cancer statistics CA Cancer Journal Clinicians 1993 43: 7–26
Denis L, Murphy GP . Overview of phase III trials on combined androgen treatment in patients with metastatic prostate cancer Cancer 1993 72: (Suppl) 3888–3895
Denmeade SR, Isaacs JT . Activation of programmed (apoptotic) cell death for the treatment of prostate cancer Advances Pharmacol 1996 35: 281–306
Tang DG, Porter AT . Target to apoptosis: a hopeful weapon for prostate cancer The Prostate 1997 32: 284–293
Basu A, Haldar S . The relationship between Bcl-2, Bax and p53: consequences for cell cycle progression and cell death Mol Human Repro 1998 12: 1099–1109
Cardillo M et al. Resistance to apoptosis and up regulation of Bcl-2 in benign prostatic hyperplasia after androgen deprivation J Urol 1999 158: 212–216
Tang DG, Li L, Chopra DP, Porter AT . Extended survivability of prostate cancer cells in the absence of trophic factors: increased proliferation, evasion of apoptosis, and the role of apoptosis proteins Cancer Res 1998 58: 3466–3479
Carroll AG, Voeller HJ, Sugars L, Gelmann EP . p53 oncogene mutations in three human prostate cancer cell lines Prostate 1993 23: 123–134
Lin MF et al. Expression of human prostatic acid phosphatase correlates with androgen-stimulated cell proliferation in prostate cancer cell lines J Biol Chem 1998 273: 5939–5947
Mitchell S et al. Phenotypic and genotypic characterization of commonly used human prostatic cell lines Br J Uro 2000 85: 932–944
Veldscholte J et al. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens J Steroid Biochem Mol Biol 1992 41: 665–669
Veldscholte J et al. Unusual specificity of the androgen receptor in the human prostate tumor cell line LNCaP: high affinity for progestagenic and estrogenic steroids Biochim Biophys Acta 1990 1052: 187–194
Gao M, Ossowski L, Ferrari AC . Activation of Rb and decline in androgen receptor protein precede retinoic acid-induced apoptosis in androgen-dependent LNCaP cells and their androgen-independent derivative J Cell Physiol 1999 179: 336–346
Navone NM et al. TabBO: a model reflecting common molecular features of androgen-independent prostate cancer Clin Cancer Res 2000 6: 1190–1197
Rokhlin OW et al. Fas-mediated apoptosis in human prostatic carcinoma cell lines Cancer Res 1997 57: 1758–1768
Blagosklonny MV et al. Taxol-induced apoptosis and phosphorylation of Bcl-2 protein involves c-Raf-1 and represents a novel c-Raf-1 signal transduction pathway Cancer Res 1996 56: 1851–1854
Haldar S, Chintapalli J, Croce CM . Taxol induces Bcl-2 phosphorylation and death of prostate cancer cells Cancer Res 1996 56: 1253–1255
Breitschopf K et al. Post-translational modification of Bcl-2 facilitates. Its proteasome-dependent degradation: molecular characterization of the involved signaling pathway Mol Cell Biol 2000 20: 1886–1896
Srivastava RK, Mi QS, Hardwick JM, Longo DL . Deletion of the loop region of Bcl-2 completely blocks paclitaxel-induced apoptosis Proc Natl Acad Sci, USA 1999 96: 3775–3780
Gross A, McDonnell JM, Korsmeyer SJ . Bcl-2 family members and the mitochondria in apoptosis Genes Devel 1999 13: 1899–1911
Adams JM, Cory S . The Bcl-2 protein family: arbiters of cell survival Science 1998 281: 1322–1326
Lebedeva I et al. Bcl-xL in prostate cancer cells: effects of overexpression and down-regulation on chemosensitivity Cancer Res 2000 60: 6052–6060
Acknowledgements
We thank Dr Nora Navone (MD Anderson Cancer Center) for providing us the MDAPCa 2A and 2B cell lines. The assistance of the Confocal Microscopy facility and the UNMC/Eppley Molecular Biology core facility is acknowledged.
This work was supported in part by grants from the Pfeiffer (Gustavus and Louise) Foundation, National Cancer Institute (CA88184), State of Nebraska (LB 595) and the Department of Biochemistry and Molecular Biology, UNMC.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rothermund, C., Kondrikov, D., Lin, MF. et al. Regulation of Bcl-2 during androgen-unresponsive progression of prostate cancer. Prostate Cancer Prostatic Dis 5, 236–245 (2002). https://doi.org/10.1038/sj.pcan.4500582
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.pcan.4500582
Keywords
This article is cited by
-
FAM3B/PANDER inhibits cell death and increases prostate tumor growth by modulating the expression of Bcl-2 and Bcl-XL cell survival genes
BMC Cancer (2018)
-
Novel gene C17orf37 in 17q12 amplicon promotes migration and invasion of prostate cancer cells
Oncogene (2009)
-
Casodex treatment induces hypoxia-related gene expression in the LNCaP prostate cancer progression model
BMC Urology (2005)
-
Androgen signaling and post-transcriptional downregulation of Bcl-2 in androgen-unresponsive prostate cancer
Prostate Cancer and Prostatic Diseases (2004)