Abstract
The serotonin transporter promoter polymorphism (5-HTTLPR) has been associated with vulnerability to stress-induced depressive symptoms and with the speed and rate of response to antidepressant treatment. The goal of the present study was to evaluate the association between the 5-HTTLPR and the functional response of the serotonin system as measured by the neuroendocrine and cerebral metabolic response to intravenous administration of the selective serotonin reuptake inhibitor citalopram in normal control subjects. Genotyping was performed for 5-HTTLPR insertion/deletion polymorphism long (l) and short (s) variant alleles. The ll genotype was compared with the combined sl+ss and with the ss genotype alone. Citalopram plasma concentrations did not differ significantly between groups. The s allele was associated with a less of an increase in prolactin and cortisol than the ll genotype. The s allele was associated with greater decreases in left frontal, precentral and middle temporal gyri compared to the ll genotype. The ll genotype was associated with greater decreases in right frontal, insula and superior temporal gyrus compared to the ss genotype. These findings suggest that 5-HTTLPR is associated with an altered functional response of the serotonin system, which may represent a neurobiologic substrate for the differential response to antidepressant treatment in late life and the emergence of neuropsychiatric symptoms in neurodegenerative disorders.
Similar content being viewed by others
INTRODUCTION
In vivo neurochemical responses measured as the behavioral and neurochemical response to acute pharmacologic interventions or the clinical response to psychotropic drug treatment demonstrate great interindividual variability. The recent identification of genetic polymorphisms for some of the primary targets of psychotropic drug action (for example, serotonin transporters for the selective serotonin reuptake inhibitors, dopamine and serotonin receptors for the antipsychotics) have enabled investigators to evaluate the potential relationship of these polymorphisms to neurochemical measures, treatment response, and side effect induction (eg Smeraldi et al, 1998; Pollock et al, 2000; Murphy et al, 2003; Kaiser et al, 2001; Segman et al, 2003). The integration of genetic methods with in vivo neurobiologic techniques (neuroendocrine and neuroimaging studies) represents a unique opportunity to understand the functional consequences of specific genetic polymorphisms, which may have implications for the understanding of the mechanisms underlying the variability in acute and chronic psychotropic drug response.
The serotonin transporter promoter polymorphism (5-HTTLPR) has been a major focus of investigation in normal function and neuropsychiatric disease. A 44-base pair insertion deletion polymorphism in the 5′ flanking regulatory region of the serotonin transporter gene with a long (l) and short (s) variant has been described (17q11.1–q12, Heils et al, 1996; Lesch et al, 1996). The transcriptional efficiency of the promoter is greater for the l allele than for the s allele. The analysis of lymphoblast cell lines cultured from subjects for the three genotypes demonstrated greater serotonin uptake, higher serotonin transporter binding and higher serotonin transporter mRNA concentrations for the ll genotype than the sl and ss genotypes (Lesch et al, 1996). The ss and sl genotypes did not differ significantly from each other. These data indicated that in vivo neuroimaging studies might reveal differences in serotonin transporter binding densities and serotonin function in the brains of ll compared to ss and sl genotypes. Some in vivo neuroimaging studies have reported greater serotonin transporter availability associated with the ll genotype compared to ss and sl genotypes (Heinz et al, 2000). However, other studies of serotonin transporter reuptake sites in platelet and neuroimaging studies have not detected such differences (eg Greenberg et al, 1999; Shioe et al, 2003; Willeit et al, 2001).
In contrast to serotonin transporter concentrations, differences in serotonin function have been associated with the 5-HTTLPR in normal controls. Decreased serotonin function has been associated with the s allele compared to the l allele based upon: (1) a blunted neuroendocrine response to fenfluramine (the serotonin reuptake inhibitor and releasing agent) and clomipramine (a tricyclic antidepressant); (2) lower platelet serotonin uptake and (3) lower concentrations of serotonin metabolites (5-hydroxyindoleacetic acid, 5-HIAA) in cerebrospinal fluid (Whale et al, 2000; Reist et al, 2001; Strickland et al, 2003; Greenberg et al, 1999; Williams et al, 2003). Consistent with these observations, neurophysiologic indices of information processing in motor and auditory cortices have demonstrated an increased responsiveness associated with the ll genotype, following acute pharmacologic increase of serotonin concentrations (citalopram administration, Strobel et al, 2003; Eichhammer et al, 2003). These data suggest that in normal function, the 5-HTTLPR may be associated with differential serotonin sensitivity and behavioral responses mediated by serotonin. A logical next step is to further evaluate the functional significance of 5-HTTLPR by using neuroimaging methods to more directly evaluate central serotonergic function in vivo.
The most direct method for in vivo neuroimaging of serotonin function is to perform serial studies with a serotonin receptor radiotracer to measure changes in receptor availability secondary to pharmacologic-induced alterations in endogenous serotonin concentrations. Such a paradigm has been developed for the dopamine system using the dopamine (D2) receptor radiotracer, [11C]raclopride and acute pharmacologic interventions of the dopamine system (eg Dewey et al, 1993; Volkow et al, 1994). The development of such a paradigm involves the validation of a radiotracer with suitable imaging properties and the availability of a selective pharmacologic agent that can be safely administered to human subjects. The available radiotracers that have been evaluated for the serotonin system do not have suitable imaging properties due to such factors, as lack of reversibility of radiotracer binding and high levels of nonspecific binding (eg Meyer et al, 1999; Smith et al, 2000; Hume et al, 2001). With respect to pharmacologic agents for the serotonin system, many of the available agents are not selective, have poor bioavailability after administration of a single dose, or have safety concerns. The most selective of the serotonin reuptake inhibitors, citalopram has been developed as a suitable selective pharmacologic agent for the serotonin system that can be administered in intravenous form safely to human subjects across the life span (Seifritz et al, 1996; Smith et al, 2002a, 2002b; Goldberg et al, 2004).
Based on these considerations, an alternative approach has been developed to measure serotonin function in vivo by combining the administration of citalopram with positron emission tomography (PET) measurements of cerebral glucose metabolism (Smith et al, 2002b). The initial studies in healthy controls demonstrated that the intravenous administration of citalopram resulted in steady-state plasma concentrations of citalopram for up to 3 h after the end of infusion and time-dependent increases in cortisol and prolactin levels. Citalopram administration decreased metabolism in the right anterior cingulate gyrus (BA 24/32), right superior (BA 9) and right middle frontal gyrus (BA 6), right parietal cortex (precuneus), right superior occipital gyrus, left thalamus and right cerebellum. Increased metabolism was observed in the left superior temporal gyrus and left occipital cortex. Similar regional and lateralized metabolic effects have been observed in other studies of the effects of serotonergic agents. For example, studies of the metabolic effects of the serotonin releasing agent and re-uptake inhibitor, fenfluramine, have observed increases in metabolism in the left hemisphere and decreases in the right hemisphere (Mann et al, 1996; Soloff et al, 2000). The lateralized effects were not originally hypothesized to occur as laterality in the distribution of serotonin transporters or receptors or the content of serotonin and its metabolites has not been demonstrated (Kabani et al, 1990). There is some preclinical and clinical evidence to suggest that the serotonin system in the right hemisphere has greater compensatory ability than in the left hemisphere (Mayberg et al, 1990; Mayberg and Robinson, 1998). Studies of the recovery of serotonin receptor (5-HT2A) binding after poststroke depression in human subjects and after traumatic brain injury in an animal model both demonstrate greater increases in right compared to left hemisphere binding. The regions affected by acute, citalopram administration overlap with the regions that are altered by antidepressant interventions in mid-life and geriatric depressed patients (eg Mayberg et al, 2000; Smith et al, 1999, 2002a) and also overlap with the regions activated by mood induction paradigms, as well as attentional and memory tasks (eg Liotti et al, 2000; Fletcher et al, 1995).
The purpose of the current study was to evaluate the association between 5-HTTLPR and the neuroendocrine and cerebral metabolic response to the intravenous administration of the SSRI, citalopram in normal control subjects. The neuroendocrine (prolactin and cortisol) and cerebral glucose metabolic responses to acute citalopram administration were evaluated as described previously (Smith et al, 2002b). Based on previous basic and clinical research with 5-HTTLPR, it was hypothesized that because the s allele is associated with lower serotonin function, the s allele would be associated with less of a neuroendocrine and metabolic response compared to the l allele. Specifically, it was hypothesized that the s allele would be associated with less of a reduction in metabolism in the right hemisphere in frontal and temporal cortical regions as compared to the l allele (less of the lateralized response that has been observed in normal control subjects). It was also hypothesized that the s allele would be associated with a greater reduction in cortical metabolism in the left hemisphere compared with the l allele (as observed in patients with geriatric depression). The greater left hemisphere cortical response was hypothesized to occur as such a response has been observed in the case of decreased right hemisphere response in a previous study. This differential lateralization of response was observed in the comparison of patients with late life depression to age-matched controls, a case in which the patients would be hypothesized to have lower serotonin function than the controls.
MATERIALS AND METHODS
The procedures for subject selection and screening, for the acquisition and analysis of magnetic resonance (MR) and PET scans, and for the analysis of citalopram and neuroendocrine concentrations have been described (Smith et al, 2002a, 2002b; Foglia et al, 1997). Briefly, 20 normal control subjects (mean age 33.4±12.2 years, 10 male/10 female; 19 Caucasians and one Asian) were enrolled in the study who did not have a family history, personal history or current diagnosis of psychiatric or neurological disorders, and were medically stable (including normal values on laboratory testing, negative toxicology screening). The subjects were enrolled in one of two PET imaging studies to measure changes in cerebral glucose metabolism or dopamine (D2) receptor availability after citalopram administration. The citalopram and neuroendocrine concentrations for the total sample (n=20) and the PET data for the glucose metabolism substudy (n=15) will be presented. After a complete description of the study to the subjects, written informed consent was obtained according to procedures established by the Institutional Review Board and the Radiation Safety Committee of the North Shore-Long Island Jewish Health System.
5-HTTLPR genotyping was performed in the The Robert S Boaz Center For Genomics and Human Genetics, as described previously with modifications (Edenberg and Reynolds, 1998; Pollock et al, 2000). Lymphocytes were harvested from whole blood and DNA was extracted (QIAamp; Qiagen Inc., Valencia, CA, USA). Polymerase chain reactions (PCR) were performed using the following primers: FD 5′-TGA ATG CCA GCA CCT AAC CC-3′ and RV 5′ TTC TGG TGC CAC CTA GAC GC 3′. The amplicon product for the ‘l’ allele was 515 bp, while for the ‘s’ allele was 471 bp. In total, 10 μl PCR reactions were performed with 1 × Taq reaction Buffer (Eppendorf), 1.5 mM MgCl2, 5 ng DNA, 0.25 mM dNTP containing 0.125 mM 7 deaza dGTP (Roche), 0.15 μM of each primer and 1 U Taq gold (ABI). Cycling program was as follows: Initial denaturation step at 95° for 12 min followed by 50 cycles of 95° for 45 s, 60° for 45 s, and 72.0° for 45 s. There was a final DNA elongation step at 72° for 10 min. Amplification products were resolved by electrophoresis stained with ethidium bromide staining and visualized by UV transillumination.
The PET scans were performed using a GE Advance Tomograph in the Functional Brain Imaging Laboratory at North Shore University Hospital. Briefly, the subjects underwent intravenous infusions of placebo (250 ml of saline) or citalopram (40 mg of the drug diluted in 250 ml saline) over 60 min on 2 consecutive days. The order of placebo-drug administration was not randomized. The subjects were not told about the identity of the infusion until after completion of the two study days. The study was not randomized because if the citalopram were administered first, there might be carryover effects of the drug to the second scan. As pointed out by Kapitany et al (1999), a time interval of at least 3 weeks would be necessary between placebo and citalopram conditions due to the known carryover effects of serotonergic drugs. As this paradigm was intended for application to studies of psychiatric patients and it would not be possible to maintain patients unmedicated during such a long time interval, the citalopram was administered on the first day of study. In addition, the test–retest variability for PET studies of cerebral glucose metabolism would presumably be greater over the course of several weeks as opposed to 24–48 h.
Serum and plasma samples for assays of citalopram levels and neuroendocrine (cortisol and prolactin) concentrations, respectively, were obtained at predetermined intervals (preinfusion, end of infusion, and 15, 30 60, 90, 120 min postinfusion). The assays were performed in the Geriatric Psychopharmacology Laboratory, Western Psychiatric Institute and Clinic, University of Pittsburgh School of Medicine. The data for citalopram and neuroendocrine concentrations are shown as areas under the curve (AUC) as calculated using standard trapezoidal methods and maximum change over time (Δmax). The AUC and maximum change data were analyzed using univariate analysis of variance (ANOVA) using genotype as a between-subjects factor (ll vs ss+sl and ll vs ss).
At 30 min after the end of the infusion of placebo/citalopram, 5 mCi of [18F]fluorodeoxyglucose ([18F]-FDG) was injected as an intravenous bolus. During radiotracer uptake, subjects were maintained in a quiet, darkened room with eyes open and ear unoccluded. Subjects were positioned in the scanner. First, a 10-minute transmission scan and a 5-minute two-dimensional emission scan were acquired for photon attenuation correction. Then, a three-dimensional emission scan began at 35 min after radiotracer injection and lasted for 10 min.
Glucose metabolic rates were calculated (in ml/100 g/min) on a pixel by pixel basis as described previously (Bisaga et al, 1998; Takikawa et al, 1993). PET data processing was performed on the quantitative glucose metabolism images using the statistical parametric mapping program (SPM99, Friston et al, 1995) The glucose metabolic rates were normalized by scaling to a common mean value across all scans using the proportional scaling option. The scans were normalized as the test–retest variability for glucose metabolism studies is greater for absolute than for normalized metabolic rates (eg Bartlett et al, 1988). The PET scans for each subject were aligned, then were spatially normalized by nonlinearly warping into Talairach space. The images were smoothed with an isotropic Gaussian kernel (FWHM 8 mm in all planes). The differences in response (placebo/citalopram) were compared between-groups (ll vs ss+sl and ll vs ss) using the multigroup: conditions and covariates option in SPM99. The variance was calculated using a voxel by voxel estimation method in SPM99. The between-group comparisons were considered significant at z⩾3.08, p⩽0.001, uncorrected for multiple independent comparisons. The probability values for the cluster data that were corrected for the number of comparisons are also reported. The minimum number of voxels required for significance in the cluster was one hundred.
RESULTS
The demographic characteristics of the neuroendocrine and PET study samples are presented in Table 1. The frequencies of the l and s alleles in this sample are consistent with the population (eg Delbruck et al, 1997; Gelernter et al, 1999). For example, in Caucasians, the frequencies for ll is about 35±5% and sl/ss is about 65±5% (l is about 60% and s about 40%). The groups did not differ significantly in age; however, the mean age for the ll genotype subjects was older than for the ss+sl genotype subjects (neuroendocrine sample: F=2.42, df=2,19, p>0.05. PET sample: F=2.73, df=2,14, p>0.05). Age-related alterations in serotonin function have been shown within this age-range (eg Tauscher et al, 2001; Rosier et al, 1996). Thus, age was included as a covariate in the analyses of the citalopram concentrations, the neuroendocrine data and the glucose metabolism data. The gender distribution differed across genotypes such that the ll genotype consisted of mostly females, the combined sl+ss genotypes were balanced between the genders and the ss genotype consisted of mostly males. The citalopram concentrations and the neuroendocrine data were analyzed using gender as a covariate. For the glucose metabolism data, a separate analysis was performed to evaluate the main effect of gender, in particular, regional differences in response between the genders.
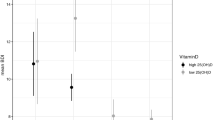
The means and standard deviations for the citalopram plasma concentrations, prolactin and cortisol AUC and the Δmax for the three groups (ll, sl+ss, ss) are shown in Table 2. The genotypes (ll compared to sl+ss) did not differ significantly in the citalopram AUC (F=0.78, df=1,19, p>0.05). For the prolactin and cortisol AUC and maximal change, the values for the ss+sl genotypes were consistently lower (26–60%) than for the ll genotype. However, the effect of genotype did not reach statistical significance for cortisol AUC or maximum change (F=0.504, df=1,19, p>0.05; F=1.08, df=1,19, p>0.05) or the prolactin AUC or maximum change (F=3.13, df=1,19, p>0.05; F=1.92, df=1,19, p>0.05). The statistically significant differences between genotypes for these variables were also not observed when other analysis methods were employed (evaluation of the log transformed data, repeated analysis of variance using the specific timepoints). The results of the ANOVAs including age as a covariate revealed a significant effect of genotype (ll compared to sl+ss) for the prolactin AUC (F=7.80, df=1,19, p<0.05) and maximum change (F=6.15, df=1,19, p<0.05), while the main effect of the covariate was not significant (p>0.05). The comparisons for the citalopram levels and cortisol measures were still not statistically significant. The analysis of the three genotypes did not reveal a statistically significant difference for these variables (p>0.05). The analyses including gender as a covariate did not reveal a significant main effect of genotype or a significant effect of gender as a covariate (p>0.05).
The SPM results of the between-group differences in the metabolic response as a function of genotype are presented in Table 3. The analyses are presented for the comparison between the ll and combined ss+sl genotypes and the comparison between the ll and ss genotypes. The results of the SPM analyses were similar whether or not age was included as a covariate. The analyses using age as a covariate are presented. The results are displayed that were statistically significant at the level of p⩽0.001 (uncorrected) at the voxel level and the statistical probabilities for the cluster level comparisons (corrected) for these voxels are shown. The combined ss+sl genotypes compared to the ll genotype demonstrated greater decreases in left superior frontal gyrus, left precentral gyrus, and left middle temporal gyrus compared to the ll genotype. The ll genotype demonstrated greater decreases in right insula and right superior temporal gyrus compared to the ss+sl genotype.
For the analyses comparing the two homozygous genotypes (ss and ll), greater decreases in metabolism in the ss compared to the ll genotype was observed in left middle frontal, left precentral, and left middle temporal cortices. Greater decreases in the ll compared to the ss genotype were observed in the right inferior frontal gyrus, right insula, and right superior temporal gyrus. Topographic maps of the representative areas of greater and lesser decrease in the ss+sl genotypes relative to the ll genotype are shown in Figure 1. The analyses of the effects of gender on the cerebral metabolic response to citalopram did not demonstrate a significant gender effect at the same statistical cutoff used in the present study in the same regions. The left and right anterior cingulate and left precuneus demonstrated greater decreases in metabolism after citalopram administration in female subjects than male subjects (p<0.01 at the uncorrected voxel level).
Alterations in cerebral glucose metabolism in ss+sl genotypes compared to ll genotype in the transaxial plane superimposed on an MR template (scaled z scores shown at a threshold of p⩽0.001). The image on the left shows greater decreases in ss+sl than ll genotypes (left superior frontal gyrus and left precentral gyrus). The image on the right shows greater decreases in ll than ss+sl genotypes (right insula and superior temporal gyrus).
DISCUSSION
The analysis of the citalopram and neuroendocrine concentrations did not reveal statistically significant differences between genotypes, except in the case of the prolactin AUC and maximum change variable when age was included as a covariate for the comparison of the ll and combined ss+sl genotypes. The general trend was for cortisol and prolactin data to be similar to that of other neuroendocrine studies, in that the ss+sl genotypes demonstrated less of response to an acute pharmacologic increase of serotonin concentrations than the ll genotype (eg Reist et al, 2001; Whale et al, 2000). However, the sample size in the study may not have had sufficient statistical power to demonstrate a difference between groups in the neuroendocrine response. The mean citalopram concentrations were similar between groups, indicating that differences in drug concentrations did not contribute to the differences in the neuroendocrine and glucose metabolic variables.
Regarding the glucose metabolism data, the ss+sl genotypes demonstrated less of a right cortical metabolic response compared to the ll genotype. A greater decrease in left hemisphere cortical metabolism following citalopram administration was observed in the ss+sl genotypes compared to the ll genotype. The greater right hemisphere response was more statistically significant in comparing the ll genotype to the ss genotype as compared to the combined sl+ss genotypes. The lesser decrease in right cortical metabolism and the greater decrease in left hemisphere cortical metabolism in comparing subjects with the s allele to the ll genotype is similar to that observed in the comparison between patients with geriatric depression and age-matched controls (Smith et al, 2002a). Thus, there are similarities between the comparison of the glucose metabolic response to citalopram of the s allele to the l allele and the comparison between patients with geriatric depression and controls. Depressive disorders, particularly in the elderly, may be associated with diminished serotonergic function (as reviewed by Meltzer et al, 1998) and the s allele has been associated with relatively lower serotonin function than the l allele. In both cases (the comparison of depressed patients to controls and the comparison of genotypes), the different cortical lateralized response might reflect a compensatory response to relatively decreased serotonin function. Other neurobiologic measures have demonstrated differential lateralized responses in comparing depressed patents to controls. For example, using neurophysiologic measures, frontal cortical asymmetries have been associated with depression, the response to antidepressant treatment, vulnerability to depression, and affective styles (Davidson, 1992; Davidson et al, 2003; Diego et al, 2001; Coan and Allen, 2003). There is evidence that these frontal asymmetries may be inheritable (Field et al, 1995). As both depressed geriatric patients and subjects with the s allele had similar lateralized patterns of response relative to controls to a serotonergic challenge, it is possible that this asymmetric sensitivity to serotonin may underlie a genetic vulnerability to depression and to slower antidepressant response.
In regard to the interpretation of the neurochemical mechanisms underlying the glucose metabolic alterations, it is important to consider that the metabolic changes secondary to citalopram administration represent the net primary (serotonin) and secondary (other neurotransmitters and neuromodulators) neurochemical effects. In addition to increasing serotonin concentrations, acute citalopram administration has been shown to increase the concentrations of other neurotransmitters including glutamate, norepinephrine, dopamine, and acetylcholine (Kreiss et al, 1993; Golembiowska and Dziubina, 2000; Invernizzi et al, 1997; Lucas et al, 2000; Mateo et al, 2000; Hilgert et al, 2000). The metabolic effects are observed primarily in the heteromodal association cortices for which interactions of serotonin and glutamate in neocortical pyramidal cells within these regions are well documented (as reviewed by Marek and Aghajanian, 1998). Thus, the regional alterations in cortical glucose metabolism observed are most consistent with the secondary effects of increased serotonin concentrations on glutamate. Future analyses of genotypes for these other neurotransmitter systems, including the glutamate system, and other potential sites involved in the antidepressant response (5-HT1A) in combination with 5-HTTLPR may explain a greater degree of the variability observed in the metabolic response to citalopram.
With respect to the role of the 5-HTTLPR in neuropsychiatric disease, there are several cases in which the s allele has been related to antidepressant treatment response and aspects of behavior, instances in which serotonin dysregulation has been hypothesized as a neurochemical substrate. The s allele has been associated with a slower response to selective serotonin reuptake inhibitor treatment (SSRI) in geriatric depression (Pollock et al, 2000) and a poorer outcome to SSRI treatment in midlife depressed patients in some studies (eg Smeraldi et al, 1998; Rausch et al, 2002, as reviewed by Lotrich et al, 2001). A prospective, longitudinal study demonstrated that one or two copies of the s allele was associated with greater stress-induced depressive symptoms and suicidality compared to the ll genotype (Caspi et al, 2003). Also, the s allele has been associated with an increased activation response in the right amygdala in response to fearful stimuli compared to the ll genotype (Hariri et al, 2002). Altered serotonin function associated with 5-HTTLPR, as suggested by the data reviewed in the Introduction, as well as the findings of the present study, may represent a neurochemical substrate that might account in part for these findings.
The 5-HTTLPR may have an impact on the course of the normal aging process, in terms of the capacity for adaptation to age-related alterations in serotonin function, as well as the emergence of behavioral symptoms in neurodegenerative disease. As discussed, there is evidence for an increase in depressive symptoms and suicidality in response to stress associated with the s allele (Caspi et al, 2003). It is possible, then, that an increase in vulnerability to depression in late life, secondary to such factors as bereavement and medical illness, may be associated with the s allele. The potential ‘neurotoxic’ effects of stress, which may be related to neurodegenerative changes in the brain have been hypothesized to be mediated by glucocorticoid modulation by the serotonin system (Bremner, 1999). The degree of susceptibility to potential stress-induced neurodegenerative changes may be associated with 5-HTTLPR. In fact, differential susceptibility to glucocorticoid increases in serotonin transporter expression have been associated with 5-HTTLPR (Glatz et al, 2003). The s allele may be also associated with a reduced capacity for neuroplasticity. For example, reduced brain-derived neurotrophic factor (BDNF) modulation of serotonin function in lymphoblasts has been associated with the s allele (Mossner et al, 2000). Behavioral disturbances observed in neurodegenerative disorders such as agitation and psychosis in Alzheimer's Disease, and depression in Parkinson's Disease have been associated with 5-HTTLPR (Sukonick et al, 2001; Sweet et al, 2001; Mossner et al, 2001). Further studies to evaluate the functional serotonergic correlates of serotonin polymorphisms (eg 5-HTTLPR, 5-HT1A, and 5-HT2A receptor genotypes) may lead to the development of protective and therapeutic strategies for the improved clinical management of these symptoms associated with late life.
The present study is clearly limited by the relatively small sample size. These findings await replication in a larger subject sample, as do other studies that have reported such findings with a similar sample size. The difference in age and the lack of gender matching could potentially limit the ability to detect a between-genotype difference. However, analyses of a larger sample of normal volunteer across the life span did not reveal gender or age effects that were similar in nature to the results observed in the present study and these variables were included in the analyses of the citalopram concentration, neuroendocrine, and neurometabolic data. The groups were reasonably well matched for ethnicity (Goldberg et al, 2004). The one Asian subject was included in the analysis as the only evidence for a differential serotonergic response in Asians was a single paper reporting a more rapid antidepressant response associated with the s allele, in contrast to l allele in studies of non-Asian patients (Kim et al, 2000; eg Pollock et al, 2000). This finding has not been replicated in other studies in Asian samples (eg Yu et al, 2002). One strategy that could be implemented in future studies would be to pre-select subjects based upon genotype in order to more closely match subjects based on age and gender. The other factor that might contribute to the results obtained is that the order of placebo/citalopram administration was not randomized and the differences in metabolism between scans may be attributable to differences in levels of habituation to the scanning procedures or anxiety (Stapleton et al, 1997; Schmidt et al, 1996). Nonetheless, significant and interpretable differences were observed in the neuroendocrine and PET data that were consistent with the literature. These data provide preliminary evidence of a decreased serotonergic response associated with the s allele using in vivo neuroimaging methods. The integration of genetic with dynamic, neuropharmacological imaging methods provides a unique opportunity to evaluate the functional significance of polymorphisms of neurotransmitter transporters and receptors that will have important implications for interpreting the variability in the behavioral and neurochemical response to acute pharmacologic interventions, as well as in understanding the neurobiological substrates of treatment response variability.
References
Bartlett EJ, Brodie JD, Wolf AP, Christman DR, Laska E, Meissner M (1988). Reproducibility of cerebral glucose metabolic measurements in resting human subjects. J Cerebr Blood Flow Metab 8: 502–512.
Bisaga A, Katz JL, Antonini A, Wright CE, Margouleff C, Gorman JM et al (1998). Cerebral glucose metabolism in women with panic disorder. Am J Psychiatry 155: 1178–1183.
Bremner JD (1999). Does stress damage the brain? Biol Psychiatry 45: 797–805.
Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H et al (2003). Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301: 386–389.
Coan JA, Allen JJB (2003). Frontal EEG asymmetry and behavioral activation and inhibition systems. Psychophysiology 40: 106–114.
Davidson RJ (1992). Emotion and affective style: hemisphere substrates. Psychol Sci 3: 39–43.
Davidson RJ, Irwin W, Anderle MJ, Kalin NH (2003). The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry 160: 64–75.
Delbruck SJ, Wendel B, Grunewald I, Sander T, Morris-Rosendahl D, Crocq MA et al (1997). A novel allelic variant of the human serotonin transporter gene regulatory polymorphism. Cytogenet Cell Genet 79: 214–220.
Dewey SL, Smith GS, Logan J, Brodie JD, Fowler JS, Wolf AP (1993). Striatal binding of the PET ligand [11C]-raclopride is altered by drugs that modify synaptic dopamine levels. Synapse 13: 350–356.
Diego MA, Field T, Hernandez-Rief M (2001). CES-D depression scores are correlated with frontal EEG alpha asymmetry. Depression Anxiety 13: 32–37.
Edenberg HJ, Reynolds J (1998). Improved method for detecting the long and short promoter alleles of the serotonin transporter gene HTT (SLC6A4). Psychiatric Genet 8: 193–195.
Eichhammer P, Langguth B, Wiegand R, Kharraz A, Frick U, Hajak G (2003). Allelic variation in the serotonin transporter promoter affects neuromodulatory effects of a selective serotonin transporter reuptake inhibitor (SSRI). Psychopharmacology 166: 294–297.
Field T, Fox NA, Pickens J, Nawrocki T (1995). Relative right frontal EEG activation in 3- to 6-month-old infants of ‘depressed’ mothers. Dev Psychol 31: 358–363.
Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ (1995). The mind's eye—precuneus activation in memory-related imagery. Neuroimage 2: 195–200.
Foglia JP, Pollock BG, Kirshner MA, Rosen J, Sweet R, Mulsant B (1997). Plasma levels of citalopram enantiomers and metabolites in elderly patients. Psychopharmacol Bull 33: 109–112.
Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ (1995). Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapping 2: 189–210.
Gelernter J, Cubells JF, Kidd JR, Pakstis AJ, Kidd KK (1999). Population studies of polymorphisms of the serotonin transporter protein gene. Am J Med Genet 88: 61–66.
Glatz K, Massner R, Heils A, Lesch KP (2003). Glucocorticoid-regulated human serotonin transporter (5-HTT) expression is modulated by the 5-HTT gene-promotor-linked polymorphic region. J Neurochem 86: 1072–1078.
Goldberg S, Smith G, Ma Y, Kramer E, Pollock B, Eidelberg D (2004). The effects of normal aging on serotonin modulation of cerebral glucose metabolism. Neurobiol Aging 25: 167–174.
Golembiowska K, Dziubina A (2000). Effect of acute and chronic administration of citalopram on glutamate and aspartate release in the rat prefrontal cortex. Polish J Pharmacol 52: 441–448.
Greenberg BD, Tolliver TJ, Huang SJ, Li Q, Bengel D, Murphy DL (1999). Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am J Med Genet 88: 83–87.
Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D et al (2002). Serotonin transporter genetic variation and the response of the human amygdala. Science 297: 400–403.
Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D et al (1996). Allelic variation of human serotonin transporter gene expression. J Neurochem 66: 2621–2624.
Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D et al (2000). A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry 47: 643–649.
Hilgert M, Buchholzer M, Jeltsch H, Kelche C, Cassel JC, Klein J (2000). Serotonergic modulation of hippocampal acetylcholine release after long-term neuronal grafting. Neuroreport 11: 3063–3065.
Hume S, Hirani E, Opacka-Juffry J, Myers R, Townsend C, Pike V et al (2001). Effect of 5-HT on binding of [11C] WAY 100635 to 5-HT1A receptors in rat brain, assessed using in vivo microdialysis and PET after fenfluramine. Synapse 41: 150–159.
Invernizzi R, Velasco C, Bramante M, Longo A, Samanin R (1997). Effect of 5-HT1A receptor antagonists on citalopram-induced increase in extracellular serotonin in the frontal cortex, striatum and dorsal hippocampus. Neuropharmacology 36: 467–473.
Kabani NJ, Reader TA, Dykes RW (1990). Monoamines and their metabolites in somatosensory, visual, and cingulate cortices of adult rat: differences in content and lack of sidedness. Neurochem Res 15: 1031–1036.
Kaiser R, Tremblay PB, Schmider J, Henneken M, Dettling M, Muller-Oerlinghausen B et al (2001). Serotonin transporter polymorphisms: no association with response to antipsychotic treatment, but associations with the schizoparanoid and residual subtypes of schizophrenia. Mol Psychiatry 6: 179–185.
Kapitany T, Schindl M, Schindler S, Henelmann B, Fureder T, Barnas C et al (1999). The citalopram challenge test in patients with major depression and in healthy controls. Psychiatry Res 88: 75–88.
Kim DK, Lim SW, Lee S, Sohn SE, Kim S, Hahn CG et al (2000). Serotonin transporter gene polymorphism and antidepressant response. Neuroreport 11: 215–219.
Kreiss DS, Wieland S, Lucki I (1993). The presence of a serotonin uptake inhibitor alters pharmacological manipulations of serotonin release. Neuroscience 52: 295–301.
Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S et al (1996). Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274: 1527–1531.
Lotrich FE, Pollock BG, Ferrell RE (2001). Polymorphism of the serotonin transporter: implications for the use of selective serotonin reuptake inhibitors. Am J PharmacoGenomics 1: 153–164.
Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT (2000). Differential limbic-cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol Psychiatry 48: 30–42.
Lucas G, De Deurwaerdere P, Porras G, Spampinato U (2000). Endogenous serotonin enhances the release of dopamine in the striatum only when nigro-striatal dopaminergic transmission is activated. Neuropharmacology 39: 1984–1995.
Mann JJ, Malone KM, Diehl DJ, Perel J, Nichols TE, Mintun MA (1996). Positron emission tomographic imaging of serotonin activation effects on prefrontal cortex in healthy volunteers. J Cerebr Blood Flow Metab 16: 418–426.
Marek GJ, Aghajanian GK (1998). The electrophysiology of prefrontal serotonin systems: therapeutic implications for mood and psychosis. Biol Psychiatry 44: 1118–1127.
Mateo Y, Ruiz-Ortega JA, Pineda J, Ugedo L, Meana JJ (2000). Inhibition of 5-hydroxytryptamine reuptake by the antidepressant citalopram in the locus coeruleus modulates the rat brain noradrenergic transmission in vivo. Neuropharmacology 39: 2036–2043.
Mayberg HS, Brannan SK, Tekell JL, Silva A, Mahurin RK, McGinnis S et al (2000). Regional Metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 48: 830–843.
Mayberg HS, Moran TH, Robinson RG (1990). Remote lateralized changes in cortical [3H]spiperone binding following focal frontal cortex lesions in the rat. Brain Res 516: 127–131.
Mayberg HS, Robinson RG (1998). PET imaging of cortical S2 serotonin receptors after stroke: lateralized changes and relationship to depression. Am J Psychiatry 145: 937–943.
Meltzer CC, Smith G, DeKosky ST, Pollock BG, Mathis CA, Moore RY et al (1998). Serotonin in aging, late-life depression, and Alzheimer's disease: the emerging role of functional imaging. Neuropsychopharmacology 18: 407–430.
Meyer JH, Cho R, Kennedy S, Kapur S (1999). The effects of single dose nefazodone and paroxetine upon 5-HT2A binding potential in humans using [18F]-setoperone PET. Psychopharmacology (Berlin) 144: 279–281.
Mossner R, Daniel S, Albert D, Heils A, Okladnova O, Schmitt A et al (2000). Serotonin transporter function is modulated by brain-derived neurotrophic factor (BDNF) but not nerve growth factor (NGF). Neurochem Int 36: 197–202.
Mossner R, Henneberg A, Schmitt A, Syagailo YV, Grassle M, Hennig T et al (2001). Allelic variation of serotonin transporter expression is associated with depression in Parkinson's disease. Mol Psychiatry 6: 350–352.
Murphy Jr GM, Kremer C, Rodrigues HE, Schatzberg AF (2003). Pharmacogenetics of antidepressant medication intolerance. Am J Psychiatry 160: 1830–1835.
Pollock BG, Ferrell RE, Mulsant BH, Mazumdar S, Miller M, Sweet RA et al (2000). Allelic variation in the serotonin transporter promoter affects onset of paroxetine treatment response in late-life depression. Neuropsychopharmacology 23: 587–590.
Rausch JL, Johnson ME, Fei YJ, Li JQ, Shendarkar N, Hobby HM et al (2002). Initial conditions of serotonin transporter kinetics and genotype: influence on SSRI treatment trial outcome. Biol Psychiatry 51: 723–732.
Reist C, Mazzanti C, Vu R, Tran D, Goldman D (2001). Serotonin transporter promoter polymorphism is associated with attenuated prolactin response to fenfluramine. Am J Med Genet 105: 363–368.
Rosier A, Dupont P, Peuskens J, Bormans G, Vandenberghe R, Maes M et al (1996). Visualisation of loss of 5-HT2A receptors with age in healthy volunteers using [18F]altanserin and positron emission tomographic imaging. Psychiatry Res 68: 11–22.
Schmidt ME, Ernst M, Matochik JA, Maisog JM, Pan BS, Zametkin AJ et al (1996). Cerebral glucose metabolism during pharmacologic studies: test–retest under placebo conditions. J Nucl Med 37: 1142–1149.
Segman RH, Goltser T, Heresco-Levy U, Finkel B, Shalem R, Schlafman M et al (2003). Association of dopaminergic and serotonergic genes with tardive dyskinesia in patients with chronic schizophrenia. Pharmacogenomics J 3: 277–283.
Seifritz E, Baumann P, Muller MJ, Annen O, Amey M, Hemmeter U et al (1996). Neuroendocrine effects of a 20-mg citalopram infusion in healthy males. A placebo-controlled evaluation of citalopram as 5-HT function probe. Neuropsychopharmacology 14: 253–263.
Shioe K, Ichimiya T, Suhara T, Takano A, Sudo Y, Yasuno F et al (2003). No association between genotype of the promoter region of serotonin transporter gene and serotonin transporter binding in human brain measured by PET. Synapse 48: 184–188.
Smeraldi E, Zanardi R, Benedetti F, Di Bella D, Perez J, Catalano M (1998). Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol Psychiatry 3: 508–511.
Smith G, Kramer E, Hermann C, Goldberg S, Ma Y, Dhawan V et al (2002a). Serotonin modulation of cerebral glucose metabolism in geriatric depression. Am J Geriatr Psychiatry 10: 715–723.
Smith G, Ma Y, Dhawan V, Gunduz H, Carbon M, Kirshner M et al (2002b). Serotonin modulation of cerebral glucose metabolism measured with positron emission tomography (PET) in human subjects. Synapse 45: 105–112.
Smith GS, Kirschner MA, Soriso D, Lagrissi-Thode F, Proper SM, Pollock BG (2000). Evaluation of citalopram as a pharmacologic intervention of the serotonin system. Biol Psychiatry 47(Suppl. 1): 99.
Smith G, Reynolds C, Pollock B, Derbyshire S, Nofzinger E, Dew M et al (1999). Cerabral glucose metabolic response to combined total sleep deprivation and antidepressant treatment in geriatric depression. Am J Psychiatry 156: 683–689.
Soloff PH, Meltzer CC, Greer PJ, Constantine D, Kelly TM (2000). A fenfluramine-activated FDG-PET study of borderline personality disorder. Biol Psychiatry 47: 540–547.
Stapleton JM, Morgan MJ, Liu X, Yung BC, Phillips RL, Wong DF et al (1997). Cerebral glucose utilization is reduced in second test session. J Cerebr Blood Flow Metab 17: 704–712.
Strickland PL, John S, Payton A, Worthington J, Ollier WE, Deakin JF (2003). Do genetic polymorphisms of serotonin (5-HT) neurotransmission influence function in humans? Am J Med Genet 120A: 566–567.
Strobel A, Debener S, Schmidt D, Hunnerkopf R, Lesch KP, Brocke B (2003). Allelic variation in serotonin transporter function associated with the intensity dependence of the auditory evoked potential. Am J Med Genet 118B: 41–47.
Sukonick DL, Pollock BG, Sweet RA, Mulsant BH, Rosen J, Klunk WE et al (2001). The 5-HTTPR*S/*L polymorphism and aggressive behavior in Alzheimer disease. Arch Neurol 58: 1425–1428.
Sweet RA, Pollock BG, Sukonick DL, Mulsant BH, Rosen J, Klunk WE et al (2001). The 5-HTTPR polymorphism confers liability to a combined phenotype of psychotic and aggressive behavior in Alzheimer disease. Int Psychogeriatr 13: 401–409.
Takikawa S, Dhawan V, Spetsieris P, Robeson W, Chaly T, Dahl R et al (1993). Noninvasive quantitative fluorodeoxyglucose PET studies with an estimated input function derived from a population-based arterial blood curve. Radiology 188: 131–136.
Tauscher J, Verhoeff NP, Christensen BK, Hussey D, Meyer JH, Kecojevic A et al (2001). Serotonin 5-HT1A receptor binding potential declines with age as measured by [11C]WAY-100635 and PET. Neuropsychopharmacology 24: 522–530.
Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R et al (1994). Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse 16: 255–262.
Whale R, Quested DJ, Laver D, Harrison PJ, Cowen PJ (2000). Serotonin transporter (5-HTT) promoter genotype may influence the prolactin response to clomipramine. Psychopharmacology 150: 120–122.
Willeit M, Stastny J, Pirker W, Praschak-Rieder N, Neumeister A, Asenbaum S et al (2001). No evidence for in vivo regulation of midbrain serotonin transporter availability by serotonin transporter promoter gene polymorphism. Biol Psychiatry 50: 8–12.
Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ et al (2003). Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology 28: 533–541.
Yu YW, Tsai SJ, Chen TJ, Lin CH, Hong CJ (2002). Association study of the serotonin transporter promoter polymorphism and symptomatology and antidepressant response in major depressive disorders. Mol Psychiatry 7: 1115–1119.
Acknowledgements
Supported in part by National Institute of Health: MH 01621, MH 49936, MH 57078, MH 64823, MH 01509, MH 60575, M01 RR018535 and an unrestricted educational grant from Forest Laboratories. David Bjelke, CNMT and Claude Margouleff, BS are gratefully acknowledged for their contribution to the conduct of the PET studies. Margaret Kirshner, BS and Denise Soriso, BS are gratefully acknowledged for their contribution to the analysis of the citalopram and neuroendocrine concentrations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, G., Lotrich, F., Malhotra, A. et al. Effects of Serotonin Transporter Promoter Polymorphisms on Serotonin Function. Neuropsychopharmacol 29, 2226–2234 (2004). https://doi.org/10.1038/sj.npp.1300552
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300552