Abstract
Whether peroxisome proliferator-activated receptor (PPAR) δ is a good target for the chemoprevention and/or treatment of colorectal cancer (CRC) remains controversial. Our goal was to examine PPARδ expression in multistage carcinogenesis of the colorectum and to assess the relevance of PPARδ in CRC. Immunohistochemical analysis indicated that PPARδ expression increased from normal mucosa to adenomatous polyps to CRC. In cancer tissues, the PPARδ protein was accumulated only in those cancer cells with highly malignant morphology, as represented by a large-sized nucleus, round-shaped nucleus, and presence of clear nucleoli. Interestingly, the cancer tissue often contained both PPARδ-positive and -negative areas, each retaining their respective specific morphological features. Moreover, this pattern persisted even when PPARδ-positive and -negative cells were aligned next to each other within a single cancer nest or gland and was present in the majority of CRC cases. Immunohistochemistry for Ki-67 proliferation marker showed no significant correlation between Ki-67 and PPARδ in CRC samples. Based on Western blot analysis and quantitative RT–PCR, high PPARδ protein expression correlated with high PPARδ mRNA levels. Peroxisome proliferator-activated receptor δ may have a supporting role in tumorigenesis, and the close association between PPARδ expression and malignant morphology of CRC cells suggests a pivotal role in cancer tissue.
Similar content being viewed by others
Main
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors belonging to the nuclear hormone receptor superfamily. Peroxisome proliferator-activated receptors play a role in normal physiological processes such as lipid metabolism and embryo implantation, and they have been implicated in the disease-related processes of inflammation, diabetes mellitus, and cancer (Kersten et al, 2000; Willson et al, 2000). To date, three PPAR isoforms, PPARα, PPARδ/β, and PPARγ, have been isolated (Willson et al, 2000). Various functions of the PPARα and γ isotypes have been described, such as involvement in lipid homeostasis, immunity, and cellular differentiation (Tontonoz et al, 1994; Spiegelman 1998; Kliewer et al, 1999). These two isotypes also have clinical significance in the treatment of dyslipidaemia and type II diabetes mellitus (Rangwala and Lazar, 2004). In contrast, less is known about the physiological role of the PPARδ isoform, although there is some evidence supporting its involvement in embryo implantation and development (Lim et al, 1999; Barak et al, 2002), epidermal maturation and wound healing (Di-Poi et al, 2003), and regulation of fatty acid metabolism (Wang et al, 2003).
Recent studies suggest that PPARδ may play a role in colorectal cancer (CRC). The adenomatous polyposis coli (APC) and K-ras genes are known to play a role in colorectal carcinogenesis (Vogelstein et al, 1988): PPARδ expression and/or activity increase after loss of the APC gene or activation of K-ras gene expression (He et al, 1999; Shao et al, 2002). Cyclooxygenase-2 also modulates intestinal tumorigenesis (Oshima et al, 1996), and its metabolite, prostacyclin, increases PPARδ activity in CRC cells (Gupta et al, 2000). In addition, PPARδ has also been shown to be a downstream target of APC/β-catenin/T-cell factor (TCF)-4-mediated transcriptional activation, which is a key mediator in the development of CRC (He et al, 1999). However, it is currently unclear whether PPARδ, like other downstream targets such as c-myc and cyclin D1 (He et al, 1998; Tetsu and McCormick, 1999), contributes to oncogenesis and the development of colon tumours. Several studies using Apcmin mice, designed to evaluate the role of PPARδ in colon tumour development, have produced conflicting findings. Peroxisome proliferator-activated receptorδ was found to be unnecessary for small intestinal polyp formation, but might be required for the development of large-sized intestinal polyps (Barak et al, 2002). In addition, PPARδ attenuates polyp formation in chemical and genetic models (Harman et al, 2004; Reed et al, 2004). In contrast, activation of PPARδ using a synthetic ligand increases the number and size of intestinal polyps (Gupta et al, 2004); indeed, PPARδ-deficient CRC cells can establish tumours when grown as xenografts in nude mice (Park et al, 2001). In this study, we examined expression of PPARδ in multistage carcinogenesis of the colorectum in an effort to elucidate the role of PPARδ in human CRC.
Materials and methods
Cell lines
The IEC18 intestinal cell line was a generous gift from Dr I Bernard Weinstein (Herbert Irving Comprehensive Cancer Center, College of Physicians and Surgeons, Columbia University, New York, NY, USA). They were grown in Dulbecco's modified Eagle's medium plus 10% foetal bovine serum, 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin, in 5% CO2 at 37°C.
Patients and tissue samples
The expression of PPARδ was examined by immunohistochemistry in the following set of colorectal samples: normal mucosa (n=32), adenomatous polyps (n=23), and various stages of carcinomas (n=32). Tissue samples were consecutively collected in the years 2002–2003 during surgery or during endoscopic polypectomy at the Department of Surgery, Osaka University. None of the patients had a history of family syndromes for CRC. The samples of normal mucosa were cut in the longitudinal direction, and the polyps and carcinomas were cut across the maximum diameter. These samples were fixed in buffered formalin at 4°C overnight, processed through graded ethanol solutions, and embedded in paraffin. The resected samples were used with the approval of the ethical committee of Osaka University. Adenomatous polyps were 16 tubular and seven tubulovillous adenoma.
There were 23 male and nine female CRC patients, with a mean age of 59.3 years (range, 42–80 years) at surgery. Primary tumours were distributed in the colon (n=14) and rectum (n=18). The tumours were well-differentiated adenocarcinomas (n=11), moderately differentiated adenocarcinomas (n=20), and poorly differentiated adenocarcinoma (n=1). Eleven patients had lymph node metastasis and 21 patients were node-negative. Dukes' staging classified nine patients as stage A, 10 patients as stage B, nine patients as stage C, and four patients as stage D.
Antibodies
Rabbit anti-human PPARδ polyclonal antibody (sc-7197, H-74) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). This antibody recognises amino acids 2–75 mapping at the amino-terminus of PPARδ of human origin and crossreacts with mouse and rat PPARδ. Mouse anti-human β-catenin monoclonal antibody was purchased from Transduction Laboratories (Lexington, KY, USA). The rabbit anti-human Ki-67 polyclonal antibody was purchased from DAKO (Carpinteria, CA, USA). The rabbit anti-human actin antibody was purchased from Sigma-Aldrich (St Louis, MO, USA).
Haematoxylin and eosin staining and immunohistochemistry
Tissue sections (4 μm thick) were deparaffinised in xylene, rehydrated, and stained with haematoxylin and eosin (H&E) solution. The specimens were histologically diagnosed by two pathologists from the Department of Pathology, Osaka University. For immunostaining, sections were mounted on charged glass slides, boiled for antigen retrieval, and then processed for immunohistochemistry, as described previously (Takemasa et al, 2000; Yamamoto et al, 2003), using the Vectastain ABC peroxidase kit (Vector Laboratories, Burlingame, CA, USA). In the primary antibody reaction, the slides were incubated with appropriate antibodies for 1 h at room temperature. The dilution of each antibody was 1:40 for PPARδ antibody, 1:1000 for β-catenin antibody, and 1:50 for Ki-67 antibody. For negative control, nonimmunised rabbit or mouse IgG (Vector Laboratories) or PBS alone was used as a substitute for the primary antibody to exclude possible false-positive responses from secondary antibody binding or from nonspecific binding of IgG. The entire series of samples was stained twice using separately prepared sections, and no discrepant staining results were noted.
Immunohistochemical assessment
All immunostained tissue sections were evaluated by two investigators (TO and HY). Samples were coded without indicating the clinical and pathological background of the patients. In each section, 10 high-power fields were selected, and a total of at least 1000 cells were evaluated. The cell populations exhibiting an association between PPARδ expression and malignant morphology were assessed in the same manner. The results of cytoplasmic staining were expressed as a percentage of positive cells, and the intensity of staining was estimated on a scale from 0 to 3 (negative, weak, moderate, and strong). The total score was determined by multiplication of the percentage of positive cells and staining intensity, ranging from 0 to 300, as reported previously (Krajewska et al, 1996; Shamma et al, 2000). For the assessment of nuclear expression, the percentage of positive cells was examined because staining intensity was routine.
Transduction of PPARδ complementary DNA (cDNA)
The mammalian expression vector pCMX-mPPARδ, encoding mouse PPARδ cDNA (length 1.3 kb) was a generous gift from Professor Ronald M Evans (Salk Institute, San Diego, CA, USA). A pcDNA3 vector encoding a neomycin-resistant sequence was purchased from Invitrogen (Carlsbad, CA, USA). Co-transfection was carried out with pcDNA3 and PPARδ plasmid or pCMX vector at 0.5 and 2 μg, respectively, into intestinal IEC18 cells using Lipofectin Reagent (Life Technologies Inc. Gaithersburg, MD, USA). Eight hours after transfection, cells were transferred from a 60-mm dish into a 150-mm dish and selected for 10 days in the presence of 0.9 mg ml−1 of G418 (Life Technologies).
Western blot analysis
Western blot analysis was performed as described previously (Yamamoto et al, 1999). Briefly, the protein samples (50 μg) were separated using 10% polyacrylamide gel electrophoresis, followed by electroblotting onto a polyvinylidene difluoride membrane. The membrane was incubated with the primary antibodies at the appropriate concentrations (1 μg ml−1 for PPARδ antibody, 1:1000 for actin) for 1 h. Protein bands were detected using the Amersham ECL detection system (Amersham Biosciences Corp., Piscataway, NJ, USA).
Quantitative real-time PCR for PPARδ mRNA
Total cellular RNA was extracted using TRIZOL reagent (Life Technologies Inc., Gaithersburg, MD, USA). Complementary DNA was generated from 1 μg RNA with avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI, USA). Quantitative real-time PCR was performed using LightCycler™ (Idaho Technology Inc., Salt Lake City, Utah, USA), as described previously (Yamamoto et al, 2003). Quantification data from each sample were analysed using LightCycler™ analysis software. The transcription value of PPARδ was determined by plotting on a standard curve constructed using HCT116 colon cancer cells. The amount of each transcript was normalised according to that of β-actin housekeeping gene quantified with the same sample. The primer sequences were as follows: β-actin sense, 5′-GAAAATCTGGCACCACACCTT-3′; β-actin antisense, 5′-GTTGAAGGTAGTTTCGTGGAT-3′; PPARδ sense: 5′-GTGGACCTGTCACTGTCTTGTAC-3′; and PPARδ antisense: 5′-CTTCCTCTTGGAGAAGATCAGC-3′.
Statistical analysis
Statistical analysis was performed using the StatView J-5.0. program (Abacus Concepts Inc., Berkeley, CA, USA). Associations between the discrete variables were assessed using Fisher's exact tests. Data were reported as mean±s.d., and mean values were compared using the Mann–Whitney test. P-values <0.05 were accepted as statistically significant.
Results
Validation of specificity of the anti-PPARδ antibody
Immunocytochemistry showed that PPARδ-transfected cultures displayed intense PPARδ staining in comparison to the weak PPARδ staining noted in the control cultures (Figure 1A). Western blotting using anti-PPARδ antibody showed that PPARδ-introduced cultures displayed prominent bands for the PPARδ protein compared with parental and vector control cells (Figure 1B). These results indicate that PPARδ antibody specifically reacts with the PPARδ protein.
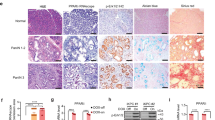
Specificity of anti-PPARδ antibody. (A) Immunocytochemistry with anti-PPARδ antibody. After selection with G418 (0.9 mg ml−1), pooled cultures from each dish were stained with anti-PPARδ antibody. Peroxisome proliferator-activated receptor δ-transfected cultures of IEC18 intestinal cells displayed intense PPARδ staining in comparison to the weak PPARδ staining noted in the control cultures. (B) Western blotting using anti-PPARδ antibody showed that PPARδ-introduced cultures displayed prominent bands for the PPARδ protein compared with parental and vector control cells.
PPARδ expression in CRC tissues
In normal colonic mucosa, PPARδ protein was detected in the epithelial cells on the luminal surface of the mucosal glands (Figure 2A). In adenomatous polyps, PPARδ was weakly expressed in eight of 23 samples (34.8%) in the cytoplasm (Figure 2B). On the other hand, all the carcinoma tissues expressed the PPARδ protein to various extents, in the cytoplasm and/or nucleus (Figures 2C and D). More than half of the cancer tissues exhibited nuclear expression at less than 10%, whereas a considerable number of the cancer tissues showed cytoplasmic PPARδ expression (Table 1). Peroxisome proliferator-activated receptorδ expression in adenomatous polyps and cancer tissues with regard to their localisation and expression extent is summarised in Table 1. The differences in cytoplasmic PPARδ expression between adenomatous polyps and cancers were significant (P<0.0001).
Immunohistochemistry for PPARδ. (A) In normal colonic mucosa, the PPARδ protein was weakly detected in the epithelial cells on the luminal surface of the mucosal glands. (B) In adenomatous polyps, PPARδ was weakly expressed in the cytoplasm. (C) Cytoplasmic expression and (D) nuclear expression in carcinoma tissues. Magnifications: A: × 50; B: × 20; C: × 100; D: × 150.
The cancer specimens were divided into two groups (high expression: n=15 (46.9%) and low expression: n=17 (53.1%)) based on the mean value of a cytoplasmic PPARδ score of 160 (see Materials and Methods regarding score determination). Comparison of these two groups showed no differences in the various clinical and pathological parameters listed in Table 2. In addition, nuclear expression level (mean value 8.3% at the cutoff point) did not correlate with clinical and pathological parameters in these two groups (data not shown).
Western blot analysis
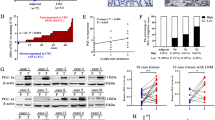
Among the above series, three CRC cases with low cytoplasmic PPARδ and four CRC cases with high cytoplasmic PPARδ were subjected to Western blot analysis to determine PPARδ protein levels (Figure 3A). Colorectal cancer samples expressed various levels of the PPARδ protein that correlated well with those detected by immunohistochemistry.
(A) Western blot analysis for the PPARδ protein. Protein levels were quantified for three CRC cases with low cytoplasmic PPARδ and four CRC cases with high cytoplasmic PPARδ levels. Peroxisome proliferator-activated receptor δ levels in cancer tissues correlated well with those detected by immunohistochemistry. (B) Quantitative RT–PCR for PPARδ mRNA. The same tissue samples used in Western blot analysis were subjected to quantitative RT–PCR for PPARδ mRNA quantification. Samples exhibiting high levels of PPARδ protein expression generally exhibited high levels of PPARδ mRNA, whereas those with low PPARδ protein levels exhibited low levels of PPARδ mRNA.
Level of PPARδ mRNA
The same tissue samples used in Western blot analysis were subjected to quantitative RT–PCR for PPARδ mRNA quantification. Samples exhibiting high expression of PPARδ protein generally also exhibited high levels of PPARδ mRNA, whereas those with low PPARδ protein levels exhibited low levels of PPARδ mRNA (Figure 3B).
Relationship between PPARδ expression and Ki-67 expression
To investigate the possible involvement of PPARδ in cell growth, we compared the expression of PPARδ and Ki-67, a cell proliferation marker, in the serial sections. Nuclear Ki-67-positive cells were localised at the proliferative zone of the normal epithelium, but were randomly distributed in cancer tissue with Ki-67 indices ranging from 22.1 to 80.0% (mean value: 49.6±15.1%). Several CRC samples displayed a concordant distribution of Ki-67-expressing cells and cells with cytoplasmic accumulation of PPARδ (Figure 4), but not nuclear PPARδ (data not shown). Analysis of all the samples, however, found no significant correlation between the cytoplasmic expression of PPARδ and Ki-67 (data not shown).
PPARδ expression and malignant morphology of CRC cells
During the course of this study, we found that the cancer cells with cytoplasmic accumulation of PPARδ often exhibited morphological features associated with a highly malignant phenotype. These features included a large nucleus, globular nuclear shape, appearance of distinct nucleolus, and loss of cellular polarity (Figure 5A). In contrast, PPARδ-negative cancer cells had morphological features associated with a low malignant phenotype, such as an oval and small nucleus without a distinct nucleolus, and preserved cellular polarity (Figure 5B). It was of interest that a cancer tissue often contained both PPARδ-positive and -negative areas, with maintenance of these respective, specific morphological features (Figure 5C). Moreover, this rule was maintained even when PPARδ-positive and -negative cells were aligned next to each other within a single cancer nest or gland (Figure 5D and E). Table 3 summarises the levels of PPARδ expression, nuclear size and shape index, and presence or absence of a distinct nucleolus in these samples.
Peroxisome proliferator-activated receptor δ expression and morphology of CRC cells. (A) PPARδ-positive cancer cells presented morphological features associated with high malignant potential including a large nucleus, globular nuclear shape, appearance of a distinct nucleolus, and loss of cellular polarity. (B) PPARδ-negative cancer cells presented a morphology (e.g., oval and small nucleus and preserved cellular polarity) associated with low malignant potential. (C) PPARδ-negative and -positive cancer cells were present within the same CRC tissue, with maintenance of their respective specific morphological features. This pattern was maintained even when PPARδ-positive and -negative cells were aligned side by side within a single cancer nest (D) or gland (E). Magnifications: A–C: × 100; D–E: upper panel × 20, lower panel × 100.
Further microscopy analysis, as indicated in the Materials and Methods, revealed that this association between PPARδ expression and malignant morphological features was a common rule in the majority of cancer samples tested. Thus, 100% of cancer cells followed the rule in the 10 CRCs, 50–99% of cancer cells followed the rule in 18 CRCs, and 1–49% of cells in three CRCs adhered to the pattern. One CRC sample did not exhibit this association.
Discussion
With regard to human tissue, increased expression of PPARδ was first reported in a small set of CRC tumours (He et al, 1999; Gupta et al, 2000). To elucidate further the expression and role of PPARδ in human colorectal tumour, we examined expression of PPARδ in multistage carcinogenesis of the colorectum and found that PPARδ expression increased from normal mucosa to adenomatous polyps to cancer tissues. Furthermore, we found that PPARδ expression was tightly associated with highly malignant morphology of colon cancer cells. Thus, the present data suggest a pivotal role of PPARδ in human CRC tissue. These findings are consistent with the recent reports that PPARδ mRNA is overexpressed in more than half of CRCs (Yang et al, 2006) and that PPARδ protein expression is elevated in adenomas in Apcmin mice and in colon tumours familial adenomatous polyposis patients (Knutsen et al, 2005). Although clinicopathological correlations were not obtained with PPARδ expression in CRC, we should emphasise that the relatively small number of CRC specimens examined may have given a low statistical power.
In spite of these indications, however, whether PPARδ is a good target for chemoprevention and/or treatment of CRC remains controversial. It has been reported that a polymorphism in PPARδ modifies the protective effects of nonsteroidal anti-inflammatory drugs on colorectal adenomas (Siezen et al, 2006), but other investigators have not reached the same conclusions in the context of CRC (McGreavey et al, 2005). As mentioned in the Introduction, PPARδ was found to be unnecessary for small intestinal polyp formation (Barak et al, 2002), but PPARδ attenuated polyp formation in chemical and genetic models (Harman et al, 2004; Reed et al, 2004). By contrast, it has been reported that inactivation of the PPARδ gene results in reduced tumorigenicity and in vivo growth of HCT116 colon cancer cells (Park et al, 2001) and that a specific PPARδ agonist enhanced in vivo growth of intestinal adenoma of Apcmin mice (Gupta et al, 2004). Moreover, a decrease in PPARδ expression by nitric-oxide-donating aspirin isomers was found to be proportional to their tumour inhibitory effects in Apcmin mice (Ouyang et al, 2006).
A recent report by Wang et al might be a clue to the puzzle. They reported that prostaglandin E2-mediated enhancement of intestinal adenoma of Apcmin mice was negated in Apcmin/PPARδ−/− mice (Wang et al, 2004a, 2004b). Peroxisome proliferator-activated receptorδ may mediate the antiapoptotic effect through activation by prostacyclin (PGI(2)), a major prostaglandin with antiapoptotic activity (Gupta et al, 2000; Cutler et al, 2003). There is also cumulative evidence regarding the antiapoptotic effects of PPARδ in keratinocyte and colon cancer cells (Michalik et al, 2001; Di-Poi et al, 2002; Shureiqi et al, 2003; Gupta et al, 2004). These findings suggest that PPARδ may play a certain tumour-promoting role in intestinal tumours or CRC cells by modulating cell survival and apoptosis, which is in line with our observation that PPARδ was exclusively expressed in those CRC cells that also exhibited highly malignant morphology.
Cellular atypia is the pathological hallmark for estimating the malignant potential of lesions. Studies with large numbers of CRC patients (N=343, 100, 90, and 64, respectively) (Ambros et al, 1990; Mitmaker et al, 1991; Fernandez-Lopez et al, 1999; Ikeguchi et al, 1999) have shown that the nuclear area, the large maximum nucleus diameter, or nuclear shape index, when determined with the aid of nuclear morphometry, is associated with cancer metastasis or poor prognosis. During the current study, we found that PPARδ-expressing cancer cells often presented such nuclear features, whereas PPARδ-negative cells did not. Surprisingly, this association was found in the majority of CRC cases and was maintained even when PPARδ-positive cells and PPARδ-negative cells were positioned next to each other. These findings indicate that cytoplasmic accumulation of PPARδ could be a sensitive marker of CRC cells with the potential for high malignancy. Recently, Hinoi et al showed that loss of CDX2 was a marker for large-cell minimally differentiated carcinomas of the colon (Hinoi et al, 2001), and we are not aware of other molecular markers tightly associated with CRC cell morphology.
PPARδ is known as a nuclear receptor, and we indeed found that introduction of PPARδ cDNA resulted in nuclear expression in IEC18 cells, whereas immunohistochemistry showed cytoplasmic accumulation of PPARδ in CRC tissues. We believe that the latter findings do not represent nonspecific binding of the PPARδ antibody in tissue samples; β-catenin staining in the same tumour series indicated that cytoplasmic PPARδ was selectively induced in CRC cells, possibly as a result of aberrant accumulation of oncogenic β-catenin (data not shown, our unpublished data). Therefore, it is postulated that cytoplasmic accumulation of PPARδ may be necessary for the proteins to be available for their nuclear role whenever required. It is also possible that nuclear PPARδ might be present at a low level but was not detectable because of the limited sensitivity of the immunohistochemical analysis.
Our immunohistochemical study of Ki-67 and PPARδ did not identify positive effects of PPARδ on in vivo cell growth. We found by in vitro assay that the growth of intestinal cells was stimulated by the introduction of PPARδ cDNA (data not shown, our unpublished data), findings that are consistent with other reports that PPARδ enhances the in vitro growth of breast and prostate cancer cells (Stephen et al, 2004). These results suggest that other positive and negative regulators could be simultaneously exerting their effects on cell growth.
In conclusion, the present study using CRC tissue samples showed that PPARδ expression increased during multistage carcinogenesis. Our data suggest that the association of PPARδ with CRC malignant cellular morphology suggests a pivotal role for PPARδ in these cells.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ambros RA, Pawel BR, Mescheryakov I, Kotrotsios J, Lambert WC (1990) Nuclear morphometry a prognostic indicator in colorectal carcinoma resected for cure. Anal Quant Cytol Histol 12: 172–176
Barak Y, Liao D, He W, Ong ES, Nelson MC, Olefsky JM, Boland R, Evans RM (2002) Effects of peroxisome proliferator-activated receptor δ on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci USA 99: 303–308
Cutler NS, Graves-Deal R, LaFleur BJ, Gao Z, Boman BM, Whitehead RH, Terry E, Morrow JD, Coffey RJ (2003) Stromal production of prostacyclin confers an antiapoptotic effect to colonic epithelial cells. Cancer Res 63: 1748–1751
Di-Poi N, Michalik L, Tan NS, Desvergne B, Wahli W (2003) The anti-apoptotic role of PPARβ contributes to efficient skin wound healing. J Steroid Biochem Mol Biol 85: 257–265
Di-Poi N, Tan NS, Michalik L, Wahli W, Desvergne B (2002) Antiapoptotic role of PPARβ in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol Cell 10: 721–733
Fernandez-Lopez F, Paredes-Cotore JP, Cadarso-Suarez C, Forteza-Vila J, Puente-Dominguez JL, Potel-Lesquereux J (1999) Prognostic value of nuclear morphometry in colorectal cancer. Dis Colon Rectum 42: 386–392
Gupta RA, Tan J, Krause WF, Geraci MW, Willson TM, Dey SK, DuBois RN (2000) Prostacyclin-mediated activation of PPARδ in colorectal cancer. Proc Natl Acad Sci USA 97: 13275–13280
Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN (2004) Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-δ accelerates intestinal adenoma growth. Nat Med 10: 245–247
Harman FS, Nicol CJ, Marin HE, Ward JM, Gonzalez FJ, Peters JM (2004) Peroxisome proliferator-activated receptor-δ attenuates colon carcinogenesis. Nat Med 10: 481–483
He TC, Chan TA, Vogelstein B, Kinzler KW (1999) PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell 99: 335–345
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c-MYC as a target of the APC pathway. Science 281: 1438–1441
Hinoi T, Tani M, Lucas PC, Caca K, Dunn RL, Macri E, Loda M, Appelman HD, Cho KR, Fearon ER (2001) Loss of CDX2 expression and microsatellite instability are prominent features of large cell minimally differentiated carcinomas of the colon. Am J Pathol 159: 2239–2248
Ikeguchi M, Sakatani T, Endo K, Makino M, Kaibara N (1999) Computerized nuclear morphometry is a useful technique for evaluating the high metastatic potential of colorectal adenocarcinoma. Cancer 86: 1944–1951
Kersten S, Desvergne B, Wahli W (2000) Roles of PPARs in health and disease. Nature 405: 421–424
Kliewer SA, Lehmann JM, Milburn MV, Willson TM (1999) The PPARs and PXRs: nuclear xenobiotic receptors that define novel hormone signaling pathways. Recent Prog Horm Res 154: 345–367
Knutsen HK, Olstorn HB, Paulsen JE, Husoy T, Goverud IL, Loberg EM, Kristiansen K, Alexander J (2005) Increased levels of PPARbeta/delta and cyclin D1 in flat dysplastic ACF and adenomas in Apc(Min/+) mice. Anticancer Res 26: 445
Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, Kitada S, Reed JC (1996) Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol 148: 1567–1576
Lim H, Gupta RA, Ma WG, Paria BC, Moller DE, Morrow JD, DuBois RN, Trzaskos JM, Dey SK (1999) Cyclooxygenase-2 derived prostacyclin mediates embryo implantation in the mouse via PPARδ. Genes Dev 13: 1561–1574
McGreavey LE, Turner F, Smith G, Boylan K, Timothy Bishop D, Forman D, Roland Wolf C, Barrett JH, Colorectal Cancer Study Group (2005) No evidence that polymorphisms in CYP2C8, CYP2C9, UGT1A6, PPARdelta and PPARgamma act as modifiers of the protective effect of regular NSAID use on the risk of colorectal carcinoma. Pharmacogenet Genom 15: 713–721
Michalik L, Desvergne B, Tan NS, Basu-Modak S, Escher P, Rieusset J, Peters JM, Kaya G, Gonzalez FJ, Zakany J, Metzger D, Chambon P, Duboule D, Wahli W (2001) Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)alpha and PPAR beta mutant mice. J Cell Biol 154: 799–814
Mitmaker B, Begin LR, Gordon PH (1991) Nuclear shape as a prognostic discriminant in colorectal carcinoma. Dis Colon Rectum 34: 249–259
Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM (1996) Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87: 803–809
Ouyang N, Williams JL, Rigas B (2006) NO-donating aspirin isomers downregulate peroxisome proliferator-activated receptor (PPAR)delta expression in APC(min/+) mice proportionally to their tumor inhibitory effect: Implications for the role of PPARdelta in carcinogenesis. Carcinogenesis 27: 232–239
Park BH, Vogelstein B, Kinzler KW (2001) Genetic disruption of PPARδ decreases the tumorigenicity of human colon cancer cells. Proc Natl Acad Sci USA 98: 2598–2603
Rangwala SM, Lazar MA (2004) Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol Sci 25: 331–336
Reed KR, Sansom OJ, Hayes AJ, Gescher AJ, Winton DJ, Peters JM, Clarke AR (2004) PPARdelta status and Apc-mediated tumourigenesis in the mouse intestine. Oncogene 23: 8992–8996
Shamma A, Yamamoto H, Doki Y, Okami J, Kondo M, Fujiwara Y, Yano M, Inoue M, Matsuura N, Shiozaki H, Monden M (2000) Up-regulation of cyclooxygenase-2 in squamous carcinogenesis of the esophagus. Clin Cancer Res 6: 1229–1238
Shao J, Sheng H, DuBois RN (2002) Peroxisome proliferator-activated receptors modulate K-Ras-mediated transformation of intestinal epithelial cells. Cancer Res 62: 3282–3288
Shureiqi I, Jiang W, Zuo X, Wu Y, Stimmel JB, Leesnitzer LM, Morris JS, Fan HZ, Fischer SM, Lippman SM (2003) The 15-lipoxygenase-1 product 13-S-hydroxyoctadecadienoic acid down-regulates PPAR-delta to induce apoptosis in colorectal cancer cells. Proc Natl Acad Sci USA 100: 9968–9973
Siezen CL, Tijhuis MJ, Kram NR, van Soest EM, de Jong DJ, Fodde R, van Kranen HJ, Kampman E (2006) Protective effect of nonsteroidal anti-inflammatory drugs on colorectal adenomas is modified by a polymorphism in peroxisome proliferator-activated receptor delta. Pharmacogenet Genom 16: 43–50
Spiegelman BM (1998) PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes 47: 507–514
Stephen RL, Gustafsson MC, Jarvis M, Tatoud R, Marshall BR, Knight D, Ehrenborg E, Harris AL, Wolf CR, Palmer CN (2004) Activation of peroxisome proliferator-activated receptor delta stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Res 64: 3162–3170
Takemasa I, Yamamoto H, Sekimoto M, Ohue M, Noura S, Miyake Y, Matsumoto T, Aihara T, Tomita N, Tamaki Y, Sakita I, Kikkawa N, Matsuura N, Shiozaki H, Monden M (2000) Overexpression of CDC25B phosphatase as a novel marker of poor prognosis of human colorectal carcinoma. Cancer Res 60: 3043–3050
Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398: 422–426
Tontonoz P, Hu E, Spiegelman BM (1994) Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 79: 1147–1156
Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL (1988) Genetic alterations during colorectal-tumor development. N Engl J Med 319: 525–532
Wang D, Mann JR, DuBois RN (2004a) WNT and cyclooxygenase-2 cross-talk accelerates adenoma growth. Cell Cycle 3: 1512–1515
Wang D, Wang H, Shi Q, Katkuri S, Walhi W, Desvergne B, Das SK, Dey SK, DuBois RN (2004b) Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell 6: 285–295
Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM (2003) Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell 113: 159–170
Willson TM, Brown PJ, Sternbach DD, Henke BR (2000) The PPARs: from orphan receptors to drug discovery. J Med Chem 43: 527–550
Yamamoto H, Kondo M, Nakamori S, Nagano H, Wakasa K, Sugita Y, Chang-De J, Kobayashi S, Damdinsuren B, Dono K, Umeshita K, Sekimoto M, Sakon M, Matsuura N, Monden M (2003) JTE-522, a cyclooxygenase-2 inhibitor, is an effective chemopreventive agent against rat experimental liver fibrosis. Gastroenterology 125: 556–571
Yamamoto H, Soh JW, Shirin H, Xing WQ, Lim JT, Yao Y, Slosberg E, Tomita N, Schieren I, Weinstein IB (1999) Comparative effects of overexpression of p27Kip1 and p21Cip1/Waf1 on growth and differentiation in human colon carcinoma cells. Oncogene 18: 103–115
Yang L, Zhou ZG, Luo HZ, Zhou B, Xia QJ, Tian C (2006) Quantitative analysis of PPARdelta mRNA by real-time RT-PCR in 86 rectal cancer tissues. Eur J Surg Oncol 32: 181–185
Acknowledgements
We thank Dr I Bernard Weinstein (Herbert Irving Comprehensive Cancer Center, College of Physicians and Surgeons, Columbia University, New York, NY, USA) for providing IEC18 intestinal cells. We also thank Professor Ronald M Evans (Salk Institute, San Diego, CA, USA) for providing the mammalian expression vector pCMX-mPPARδ, encoding mouse PPARδ cDNA. This work was supported by a grant for the 3rd-term Comprehensive Strategy for Cancer Control from the Ministry of Health Labor and Welfare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Takayama, O., Yamamoto, H., Damdinsuren, B. et al. Expression of PPARδ in multistage carcinogenesis of the colorectum: implications of malignant cancer morphology. Br J Cancer 95, 889–895 (2006). https://doi.org/10.1038/sj.bjc.6603343
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6603343
Keywords
This article is cited by
-
DLGAP4 acts as an effective prognostic predictor for hepatocellular carcinoma and is closely related to tumour progression
Scientific Reports (2022)
-
Targeting lipid mediators in cancer biology
Cancer and Metastasis Reviews (2018)
-
Hematopoietic knockdown of PPARδ reduces atherosclerosis in LDLR−/− mice
Gene Therapy (2016)
-
The prognostic significance of peroxisome proliferator-activated receptor β expression in the vascular endothelial cells of colorectal cancer
Journal of Gastroenterology (2014)
-
Transcription factor AP1 binds the functional region of the promoter and regulates gene expression of human PPARdelta in LoVo cell
Tumor Biology (2013)