Abstract
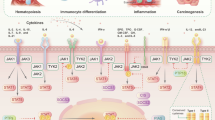
Various human malignancies are characterized by excessive activation of the Janus family of cytoplasmic tyrosine kinases (JAK) and their associated transcription factors STAT3 and STAT5. In the majority of solid tumors, this occurs in response to increased abundance of inflammatory cytokines in the tumor microenvironment prominently produced by infiltrating innate immune cells. Many of these cytokines share common receptor subunits and belong to the interleukin (IL)-6/IL-11, IL-10/IL-22 and IL-12/IL-23 families. Therapeutic inhibition of the JAK/STAT3 pathway potentially offers considerable benefit owing to the capacity of JAK/STAT3 signaling to promote cancer hallmarks in the tumor and its environment, including proliferation, survival, angiogenesis, tumor metabolism while suppressing antitumor immunity. This is further emphasized by the current successful clinical applications of JAK-specific small molecule inhibitors for the treatment of inflammatory disorders and hematopoietic malignancies. Here we review current preclinical applications for JAK inhibitors for the treatment of solid cancers in mice, with a focus on the most common malignancies emanating from oncogenic transformation of the epithelial mucosa in the stomach and colon. Emerging data with small molecule JAK-specific adenosine triphosphate-binding analogs corroborate genetic findings and suggest that interference with the JAK/STAT3 pathway may suppress the growth of the most common forms of sporadic colon cancers that arise from mutations of the APC tumor suppressor gene. Likewise inhibition of cytokine-dependent activation of the JAK/STAT3 pathway may also afford orthogonal treatment opportunities for other oncogene-addicted cancer cells that have gained drug resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Firmbach-Kraft I, Byers M, Shows T, Dalla-Favera R, Krolewski JJ . tyk2, prototype of a novel class of non-receptor tyrosine kinase genes. Oncogene 1990; 5: 1329–1336.
Kawamura M, McVicar DW, Johnston JA, Blake TB, Chen YQ, Lal BK et al. Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc Natl Acad Sci USA 1994; 91: 6374–6378.
Wilks AF, Harpur AG, Kurban RR, Ralph SJ, Zurcher G, Ziemiecki A . Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase. Mol Cell Biol 1991; 11: 2057–2065.
Ghoreschi K, Laurence A, O'Shea JJ . Janus kinases in immune cell signaling. Immunol Rev 2009; 228: 273–287.
Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood 2005; 106: 2162–2168.
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. New Engl J Med 2005; 352: 1779–1790.
Steensma DP, Dewald GW, Lasho TL, Powell HL, McClure RF, Levine RL et al. The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both "atypical" myeloproliferative disorders and myelodysplastic syndromes. Blood 2005; 106: 1207–1209.
Walters DK, Mercher T, Gu TL, O'Hare T, Tyner JW, Loriaux M et al. Activating alleles of JAK3 in acute megakaryoblastic leukemia. Cancer Cell 2006; 10: 65–75.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Chang Q, Daly L, Bromberg J . The IL-6 feed-forward loop: a driver of tumorigenesis. Semin Immunol 2014; 26: 48–53.
Schafer ZT, Brugge JS . IL-6 involvement in epithelial cancers. J Clin Invest 2007; 117: 3660–3663.
Silver JS, Hunter CA . gp130 at the nexus of inflammation, autoimmunity, and cancer. J Leuk Biol 2010; 88: 1145–1156.
Taniguchi K, Karin M . IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol 2014; 26: 54–74.
Putoczki TL, Thiem S, Loving A, Busuttil RA, Wilson NJ, Ziegler PK et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell 2013; 24: 257–271.
Onnis B, Fer N, Rapisarda A, Perez VS, Melillo G . Autocrine production of IL-11 mediates tumorigenicity in hypoxic cancer cells. J Clin Invest 2013; 123: 1615–1629.
Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Cespedes MV et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell 2012; 22: 571–584.
Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T et al. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell 2009; 15: 114–123.
Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 2012; 491: 254–258.
Chang HC, Han L, Goswami R, Nguyen ET, Pelloso D, Robertson MJ et al. Impaired development of human Th1 cells in patients with deficient expression of STAT4. Blood 2009; 113: 5887–5890.
Schindler C, Levy DE, Decker T . JAK-STAT signaling: from interferons to cytokines. J Biol Chem 2007; 282: 20059–20063.
Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005; 201: 233–240.
Murray PJ . Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol 2006; 6: 379–386.
Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 1998; 66: 5224–5231.
Pisa P, Halapi E, Pisa EK, Gerdin E, Hising C, Bucht A et al. Selective expression of interleukin 10, interferon gamma, and granulocyte-macrophage colony-stimulating factor in ovarian cancer biopsies. Proc Natl Acad Sci USA 1992; 89: 7708–7712.
Venetsanakos E, Beckman I, Bradley J, Skinner JM . High incidence of interleukin 10 mRNA but not interleukin 2 mRNA detected in human breast tumours. Br J Cancer 1997; 75: 1826–1830.
Huang M, Wang J, Lee P, Sharma S, Mao JT, Meissner H et al. Human non-small cell lung cancer cells express a type 2 cytokine pattern. Cancer Res 1995; 55: 3847–3853.
Miteva LD, Stanilov NS, Deliysky TS, Stanilova SA . Significance of -1082 A/G polymorphism of IL10 gene for progression of colorectal cancer and IL-10 expression. Tumour Biol 2014; 35: 12655–12664.
Dummer W, Becker JC, Schwaaf A, Leverkus M, Moll T, Brocker EB . Elevated serum levels of interleukin-10 in patients with metastatic malignant melanoma. Melanoma Res 1995; 5: 67–68.
Kim J, Modlin RL, Moy RL, Dubinett SM, McHugh T, Nickoloff BJ et al. IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T cell immune response. J Immunol 1995; 155: 2240–2247.
Sato T, McCue P, Masuoka K, Salwen S, Lattime EC, Mastrangelo MJ et al. Interleukin 10 production by human melanoma. Clin Cancer Res 1996; 2: 1383–1390.
Emmerich J, Mumm JB, Chan IH, LaFace D, Truong H, McClanahan T et al. IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res 2012; 72: 3570–3581.
Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S et al. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell 2011; 20: 781–796.
Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 2006; 203: 2271–2279.
McGee HM, Schmidt BA, Booth CJ, Yancopoulos GD, Valenzuela DM, Murphy AJ et al. IL-22 promotes fibroblast-mediated wound repair in the skin. J Invest Dermatol 2013; 133: 1321–1329.
Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 2009; 206: 1465–1472.
da Rocha LF Jr, Duarte AL, Dantas AT, Mariz HA, Pitta Ida R, Galdino SL et al. Increased serum interleukin 22 in patients with rheumatoid arthritis and correlation with disease activity. J Rheumatol 2012; 39: 1320–1325.
Justa S, Zhou X, Sarkar S . Endogenous IL-22 plays a dual role in arthritis: regulation of established arthritis via IFN-gamma responses. PLoS One 2014; 9: e93279.
Leipe J, Schramm MA, Grunke M, Baeuerle M, Dechant C, Nigg AP et al. Interleukin 22 serum levels are associated with radiographic progression in rheumatoid arthritis. Ann Rheum Dis 2011; 70: 1453–1457.
Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest 2008; 118: 534–544.
Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med 2009; 87: 523–536.
Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA . Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 2008; 29: 947–957.
Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 2012; 491: 259–263.
Kirchberger S, Royston DJ, Boulard O, Thornton E, Franchini F, Szabady RL et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J Exp Med 2013; 210: 917–931.
Nagakawa H, Shimozato O, Yu L, Takiguchi Y, Tatsumi K, Kuriyama T et al. Expression of interleukin-22 in murine carcinoma cells did not influence tumour growth in vivo but did improve survival of the inoculated hosts. Scand J Immunol 2004; 60: 449–454.
Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med 2011; 208: 1989–2003.
Simpson JA, Al-Attar A, Watson NF, Scholefield JH, Ilyas M, Durrant LG . Intratumoral T cell infiltration, MHC class I and STAT1 as biomarkers of good prognosis in colorectal cancer. Gut 2010; 59: 926–933.
Messina NL, Banks KM, Vidacs E, Martin BP, Long F, Christiansen AJ et al. Modulation of antitumour immune responses by intratumoural Stat1 expression. Immunol Cell Biol 2013; 91: 556–567.
Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA 1998; 95: 7556–7561.
Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001; 410: 1107–1111.
Street SE, Trapani JA, MacGregor D, Smyth MJ . Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J Exp Med 2002; 196: 129–134.
Bromberg JF, Horvath CM, Wen Z, Schreiber RD, Darnell JE Jr . Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon alpha and interferon gamma. Proc Natl Acad Sci USA 1996; 93: 7673–7678.
Chin YE, Kitagawa M, Kuida K, Flavell RA, Fu XY . Activation of t he STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol Cell Biol 1997; 17: 5328–5337.
Chin YE, Kitagawa M, Su WC, You ZH, Iwamoto Y, Fu XY . Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science 1996; 272: 719–722.
Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol 2005; 6: 722–729.
Hanada T, Kobayashi T, Chinen T, Saeki K, Takaki H, Koga K et al. IFNγ-dependent, spontaneous development of colorectal carcinomas in SOCS1-deficient mice. J Exp Med 2006; 203: 1391–1397.
Greenwood C, Metodieva G, Al-Janabi K, Lausen B, Alldridge L, Leng L et al. Stat1 and CD74 overexpression is co-dependent and linked to increased invasion and lymph node metastasis in triple-negative breast cancer. J Proteomics 2012; 75: 3031–3040.
Duarte CW, Willey CD, Zhi D, Cui X, Harris JJ, Vaughan LK et al. Expression signature of IFN/STAT1 signaling genes predicts poor survival outcome in glioblastoma multiforme in a subtype-specific manner. PLoS One 2012; 7: e29653.
Goldstein D, Laszlo J . The role of interferon in cancer therapy: a current perspective. CA Cancer J Clin 1988; 38: 258–277.
Hauschild A, Gogas H, Tarhini A, Middleton MR, Testori A, Dreno B et al. Practical guidelines for the management of interferon-alpha-2b side effects in patients receiving adjuvant treatment for melanoma: expert opinion. Cancer 2008; 112: 982–994.
Gajewski TF, Louahed J, Brichard VG . Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J 2010; 16: 399–403.
Lee SK, Seo SH, Kim BS, Kim CD, Lee JH, Kang JS et al. IFN-gamma regulates the expression of B7-H1 in dermal fibroblast cells. J Dermatol Sci 2005; 40: 95–103.
Cirillo D, Rachiglio AM, la Montagna R, Giordano A, Normanno N . Leptin signaling in breast cancer: an overview. J Cell Biochem 2008; 105: 956–964.
Colomiere M, Ward AC, Riley C, Trenerry MK, Cameron-Smith D, Findlay J et al. Cross talk of signals between EGFR and IL-6 R through JAK2/STAT3 mediate epithelial-mesenchymal transition in ovarian carcinomas. Br J Cancer 2009; 100: 134–144.
Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest 2007; 117: 3846–3856.
Yeatman TJ . A renaissance for SRC. Nat Rev Cancer 2004; 4: 470–480.
Yeh YT, Lee KT, Tsai CJ, Chen YJ, Wang SN . Prolactin promotes hepatocellular carcinoma through Janus kinase 2. World J Surg 2012; 36: 1128–1135.
Johnston PA, Grandis JR . STAT3 signaling: anticancer strategies and challenges. Molecular Interv 2011; 11: 18–26.
Brivanlou AH, Darnell JE Jr . Signal transduction and the control of gene expression. Science 2002; 295: 813–818.
Ferbeyre G, Moriggl R . The role of Stat5 transcription factors as tumor suppressors or oncogenes. Biochim Biophys Acta 2011; 1815: 104–114.
Levy DE, Darnell JE Jr . Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 2002; 3: 651–662.
O'Shea JJ, Gadina M, Schreiber RD . Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell 2002; 109: S121–S131.
Ram PT, Iyengar R . G protein coupled receptor signaling through the Src and Stat3 pathway: role in proliferation and transformation. Oncogene 2001; 20: 1601–1606.
Silva CM . Role of STATs as downstream signal transducers in Src family kinase-mediated tumorigenesis. Oncogene 2004; 23: 8017–8023.
Yu H, Pardoll D, Jove R . STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009; 9: 798–809.
Bromberg JF, Horvath CM, Besser D, Lathem WW, Darnell JE Jr . Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol 1998; 18: 2553–2558.
Kazansky AV, Rosen JM . Signal transducers and activators of transcription 5B potentiates v-Src-mediated transformation of NIH-3T3 cells. Cell Growth Differ 2001; 12: 1–7.
Odajima J, Matsumura I, Sonoyama J, Daino H, Kawasaki A, Tanaka H et al. Full oncogenic activities of v-Src are mediated by multiple signaling pathways. Ras as an essential mediator for cell survival. J Biol Chem 2000; 275: 24096–24105.
Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot RP, Jove R . Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol 1998; 18: 2545–2552.
Olayioye MA, Beuvink I, Horsch K, Daly JM, Hynes NE . ErbB receptor-induced activation of stat transcription factors is mediated by Src tyrosine kinases. J Biol Chem 1999; 274: 17209–17218.
Biscardi JS, Tice DA, Parsons SJ . c-Src receptor tyrosine kinases, and human cancer. Adv Cancer Res 1999; 76: 61–119.
Xi S, Zhang Q, Dyer KF, Lerner EC, Smithgall TE, Gooding WE et al. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J Biol Chem 2003; 278: 31574–31583.
Xi S, Zhang Q, Gooding WE, Smithgall TE, Grandis JR . Constitutive activation of Stat5b contributes to carcinogenesis in vivo. Cancer Res 2003; 63: 6763–6771.
Chen P, Levis M, Brown P, Kim KT, Allebach J, Small D . FLT3/ITD mutation signaling includes suppression of SHP-1. J Biol Chem 2005; 280: 5361–5369.
Melzner I, Bucur AJ, Bruderlein S, Dorsch K, Hasel C, Barth TF et al. Biallelic mutation of SOCS-1 impairs JAK2 degradation and sustains phospho-JAK2 action in the MedB-1 mediastinal lymphoma line. Blood 2005; 105: 2535–2542.
Melzner I, Weniger MA, Bucur AJ, Bruderlein S, Dorsch K, Hasel C et al. Biallelic deletion within 16p13.13 including SOCS-1 in Karpas1106P mediastinal B-cell lymphoma line is associated with delayed degradation of JAK2 protein. Int J Cancer 2006; 118: 1941–1944.
Tartaglia M, Niemeyer CM, Fragale A, Song X, Buechner J, Jung A et al. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat Genet 2003; 34: 148–150.
Ueda M, Ota J, Yamashita Y, Choi YL, Ohki R, Wada T et al. DNA microarray analysis of stage progression mechanism in myelodysplastic syndrome. Br J Haematol 2003; 123: 288–296.
Zhang Q, Raghunath PN, Xue L, Majewski M, Carpentieri DF, Odum N et al. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma. J Immunol 2002; 168: 466–474.
Sobti RC, Singh N, Hussain S, Suri V, Nijhawan R, Bharti AC et al. Aberrant promoter methylation and loss of Suppressor of Cytokine Signalling-1 gene expression in the development of uterine cervical carcinogenesis. Cell Oncol 2011; 34: 533–543.
Sutherland KD, Lindeman GJ, Choong DYH, Wittlin S, Brentzell L, Phillips W et al. Differential hypermethylation of SOCS genes in ovarian and breast carcinomas. Oncogene 2004; 23: 7726–7733.
Fukushima N, Sato N, Sahin F, Su GH, Hruban RH, Goggins M . Aberrant methylation of suppressor of cytokine signalling-1 (SOCS-1) gene in pancreatic ductal neoplasms. Br J Cancer 2003; 89: 338–343.
Komazaki T, Nagai H, Emi M, Terada Y, Yabe A, Jin E et al. Hypermethylation-associated inactivation of the SOCS-1 Gene, a JAK/STAT inhibitor, in human pancreatic cancers. Jpn J Clin Oncol 2004; 34: 191–194.
Liu S, Ren S, Howell P, Fodstad O, Riker AI . Identification of novel epigenetically modified genes in human melanoma via promoter methylation gene profiling. Pigment Cell Melanoma Res 2008; 21: 545–558.
Marini A, Mirmohammadsadegh A, Nambiar S, Gustrau A, Ruzicka T, Hengge UR . Epigenetic inactivation of tumor suppressor genes in serum of patients with cutaneous melanoma. J Invest Dermatol 2005; 126: 422–431.
Nagai H, Kim YS, Konishi N, Baba M, Kubota T, Yoshimura A et al. Combined hypermethylation and chromosome loss associated with inactivation of SSI-1/SOCS-1/JAB gene in human hepatocellular carcinomas. Cancer Lett 2002; 186: 59–65.
Yoshikawa H, Matsubara K, Qian G-S, Jackson P, Groopman JD, Manning JE et al. SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat Genet 2001; 28: 29–35.
Okochi O, Hibi K, Sakai M, Inoue S, Takeda S, Kaneko T et al. Methylation-mediated silencing of SOCS-1 gene in hepatocellular carcinoma derived from cirrhosis. Clin Cancer Res 2003; 9: 5295–5298.
Yoshida T, Ogata H, Kamio M, Joo A, Shiraishi H, Tokunaga Y et al. SOCS1 is a suppressor of liver fibrosis and hepatitis-induced carcinogenesis. J Exp Med 2004; 199: 1701–1707.
Qiu X, Zheng J, Guo X, Gao X, Liu H, Tu Y et al. Reduced expression of SOCS2 and SOCS6 in hepatocellular carcinoma correlates with aggressive tumor progression and poor prognosis. Mol Cell Biochem 2013; 378: 99–106.
Li Y, de Haar C, Chen M, Deuring J, Gerrits MM, Smits R et al. Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut 2010; 59: 227–235.
Li Y, Deuring J, Peppelenbosch MP, Kuipers EJ, de Haar C, van der Woude CJ . IL-6-induced DNMT1 activity mediates SOCS3 promoter hypermethylation in ulcerative colitis-related colorectal cancer. Carcinogenesis 2012; 33: 1889–1896.
He B, You L, Uematsu K, Zang K, Xu Z, Lee AY et al. SOCS-3 is frequently silenced by hypermethylation and suppresses cell growth in human lung cancer. Proc Natl Acad Sci 2003; 100: 14133–14138.
Lindemann C, Hackmann O, Delic S, Schmidt N, Reifenberger G, Riemenschneider M . SOCS3 promoter methylation is mutually exclusive to EGFR amplification in gliomas and promotes glioma cell invasion through STAT3 and FAK activation. Acta Neuropathologica 2011; 122: 241–251.
Martini M, Pallini R, Luongo G, Cenci T, Lucantoni C, Larocca LM . Prognostic relevance of SOCS3 hypermethylation in patients with glioblastoma multiforme. Int J Cancer 2008; 123: 2955–2960.
Pierconti F, Martini M, Pinto F, Cenci T, Capodimonti S, Calarco A et al. Epigenetic silencing of SOCS3 identifies a subset of prostate cancer with an aggressive behavior. Prostate 2011; 71: 318–325.
Inagaki-Ohara K, Kondo T, Ito M, Yoshimura A . SOCS inflammation, and cancer. Jak-Stat 2013; 2: e24053.
Sasi W, Sharma AK, Mokbel K . The role of suppressors of cytokine signalling in human neoplasms. Mol Biol Int 2014; 2014: 630797.
Fujitake S, Hibi K, Okochi O, Kodera Y, Ito K, Akiyama S et al. Aberrant methylation of SOCS-1 was observed in younger colorectal cancer patients. J Gastroenterol 2004; 39: 120–124.
Letellier E, Schmitz M, Baig K, Beaume N, Schwartz C, Frasquilho S et al. Identification of SOCS2 and SOCS6 as biomarkers in human colorectal cancer. Br J Cancer 2014; 111: 726–735.
Oshimo Y, Kuraoka K, Nakayama H, Kitadai Y, Yoshida K, Chayama K et al. Epigenetic inactivation of SOCS-1 by CpG island hypermethylation in human gastric carcinoma. Int J Cancer 2004; 112: 1003–1009.
Tischoff I, Hengge UR, Vieth M, Ell C, Stolte M, Weber A et al. Methylation of Socs-3 and Socs-1 in the carcinogenesis of Barrett's adenocarcinoma. Gut 2007; 56: 1047–1053.
Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest 2007; 117: 3988–4002.
Studebaker AW, Storci G, Werbeck JL, Sansone P, Sasser AK, Tavolari S et al. Fibroblasts isolated from common sites of breast cancer metastasis enhance cancer cell growth rates and invasiveness in an interleukin-6-dependent manner. Cancer Res 2008; 68: 9087–9095.
Walter M, Liang S, Ghosh S, Hornsby PJ, Li R . Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene 2009; 28: 2745–2755.
Niu G, Briggs J, Deng J, Ma Y, Lee H, Kortylewski M et al. Signal transducer and activator of transcription 3 is required for hypoxia-inducible factor-1alpha RNA expression in both tumor cells and tumor-associated myeloid cells. Mol Cancer Res 2008; 6: 1099–1105.
Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002; 21: 2000–2008.
Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene 2005; 24: 5552–5560.
Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K et al. IL-23 promotes tumour incidence and growth. Nature 2006; 442: 461–465.
Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H . IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med 2009; 206: 1457–1464.
Kamiya S, Okumura M, Chiba Y, Fukawa T, Nakamura C, Nimura N et al. IL-27 suppresses RANKL expression in CD4+ T cells in part through STAT3. Immunol Lett 2011; 138: 47–53.
Wolfle SJ, Strebovsky J, Bartz H, Sahr A, Arnold C, Kaiser C et al. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol 2011; 41: 413–424.
Qiu J, Guo X, Chen ZM, He L, Sonnenberg GF, Artis D et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity 2013; 39: 386–399.
Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 2012; 336: 1321–1325.
Upadhyay V, Poroyko V, Kim TJ, Devkota S, Fu S, Liu D et al. Lymphotoxin regulates commensal responses to enable diet-induced obesity. Nat Immunol 2012; 13: 947–953.
Zenewicz LA, Yin X, Wang G, Elinav E, Hao L, Zhao L et al. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J Immunol 2013; 190: 5306–5312.
Behnsen J, Jellbauer S, Wong CP, Edwards RA, George MD, Ouyang W et al. The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria. Immunity 2014; 40: 262–273.
Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ . Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene 2002; 21: 8404–8413.
Stephanou A, Brar BK, Knight RA, Latchman DS . Opposing actions of STAT-1 and STAT-3 on the Bcl-2 and Bcl-x promoters. Cell Death Differ 2000; 7: 329–330.
Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger WJ, Sedivy JM et al. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci USA 2001; 98: 7319–7324.
Jarnicki A, Putoczki T, Ernst M . Stat3: linking inflammation to epithelial cancer - more than a "gut" feeling? Cell Div 2010; 5: 14.
Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K et al. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res 2002; 62: 3351–3355.
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C et al. Stat3 as an oncogene. Cell 1999; 98: 295–303.
Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene 2009; 28: 2940–2947.
Yadav A, Kumar B, Datta J, Teknos TN, Kumar P . IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res 2011; 9: 1658–1667.
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF et al. A long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell 2014; 25: 666–681.
Dagvadorj A, Kirken RA, Leiby B, Karras J, Nevalainen MT . Transcription factor signal transducer and activator of transcription 5 promotes growth of human prostate cancer cells in vivo. Clin Cancer Res 2008; 14: 1317–1324.
Tan SH, Nevalainen MT . Signal transducer and activator of transcription 5 A/B in prostate and breast cancers. Endocr Relat Cancer 2008; 15: 367–390.
Stuart E, Buchert M, Putoczki T, Thiem S, Farid R, Elzer J et al. Therapeutic inhibition of Jak activity inhibits progression of gastrointestinal tumors in mice. Mol Cancer Ther 2014; 13: 468–474.
Soriano SF, Serrano A, Hernanz-Falcon P, Martin de Ana A, Monterrubio M, Martinez C et al. Chemokines integrate JAK/STAT and G-protein pathways during chemotaxis and calcium flux responses. Eur J Immunol 2003; 33: 1328–1333.
Mao YL, Li ZW, Lou CJ, Pang D, Zhang YQ . Phospho-STAT5 expression is associated with poor prognosis of human colonic adenocarcinoma. Pathol Oncol Res 2011; 17: 333–339.
Li H, Ahonen TJ, Alanen K, Xie J, LeBaron MJ, Pretlow TG et al. Activation of signal transducer and activator of transcription 5 in human prostate cancer is associated with high histological grade. Cancer Res 2004; 64: 4774–4782.
Vafaizadeh V, Klemmt P, Brendel C, Weber K, Doebele C, Britt K et al. Mammary epithelial reconstitution with gene-modified stem cells assigns roles to Stat5 in luminal alveolar cell fate decisions, differentiation, involution, and mammary tumor formation. Stem Cells 2010; 28: 928–938.
Lee TK, Man K, Poon RT, Lo CM, Yuen AP, Ng IO et al. Signal transducers and activators of transcription 5b activation enhances hepatocellular carcinoma aggressiveness through induction of epithelial-mesenchymal transition. Cancer Res 2006; 66: 9948–9956.
Wolf MJ, Hoos A, Bauer J, Boettcher S, Knust M, Weber A et al. Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell 2012; 22: 91–105.
Xiong H, Su WY, Liang QC, Zhang ZG, Chen HM, Du W et al. Inhibition of STAT5 induces G1 cell cycle arrest and reduces tumor cell invasion in human colorectal cancer cells. Lab Invest 2009; 89: 717–725.
Nevalainen MT, Xie J, Torhorst J, Bubendorf L, Haas P, Kononen J et al. Signal transducer and activator of transcription-5 activation and breast cancer prognosis. J Clin Oncol 2004; 22: 2053–2060.
Blaas L, Kornfeld JW, Schramek D, Musteanu M, Zollner G, Gumhold J et al. Disruption of the growth hormone—signal transducer and activator of transcription 5—insulinlike growth factor 1 axis severely aggravates liver fibrosis in a mouse model of cholestasis. Hepatology 2010; 51: 1319–1326.
Hosui A, Kimura A, Yamaji D, Zhu BM, Na R, Hennighausen L . Loss of STAT5 causes liver fibrosis and cancer development through increased TGF-{beta} and STAT3 activation. J Exp Med 2009; 206: 819–831.
Demaria M, Giorgi C, Lebiedzinska M, Esposito G, D'Angeli L, Bartoli A et al. A STAT3-mediated metabolic switch is involved in tumour transformation and STAT3 addiction. Aging 2010; 2: 823–842.
Vander Heiden MG, Cantley LC, Thompson CB . Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324: 1029–1033.
Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T et al. Function of mitochondrial Stat3 in cellular respiration. Science 2009; 323: 793–797.
Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC, Levy DE . Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science 2009; 324: 1713–1716.
Gough DJ, Koetz L, Levy DE . The MEK-ERK pathway is necessary for serine phosphorylation of mitochondrial STAT3 and Ras-mediated transformation. PLoS One 2013; 8: e83395.
Chueh FY, Leong KF, Yu CL . Mitochondrial translocation of signal transducer and activator of transcription 5 (STAT5) in leukemic T cells and cytokine-stimulated cells. Biochem Biophys Res Commun 2010; 402: 778–783.
Bourguignon LY, Earle C, Wong G, Spevak CC, Krueger K . Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells. Oncogene 2012; 31: 149–160.
Brinkman JA, El-Ashry D . ER re-expression and re-sensitization to endocrine therapies in ER-negative breast cancers. J Mammary Gland Biol Neoplasia 2009; 14: 67–78.
Mori T, Martinez SR, O'Day SJ, Morton DL, Umetani N, Kitago M et al. Estrogen receptor-alpha methylation predicts melanoma progression. Cancer Res 2006; 66: 6692–6698.
Yang J, Chatterjee-Kishore M, Staugaitis SM, Nguyen H, Schlessinger K, Levy DE et al. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res 2005; 65: 939–947.
Grabner B, Schramek D, Mueller KM, Moll HP, Svinka J, Hoffmann T et al. Disruption of STAT3 signalling promotes KRAS-induced lung tumorigenesis. Nat Commun 2015; 6: 6285.
Schneller D, Machat G, Sousek A, Proell V, van Zijl F, Zulehner G et al. p19(ARF) /p14(ARF) controls oncogenic functions of signal transducer and activator of transcription 3 in hepatocellular carcinoma. Hepatology 2011; 54: 164–172.
Musteanu M, Blaas L, Mair M, Schlederer M, Bilban M, Tauber S et al. Stat3 is a negative regulator of intestinal tumor progression in Apc(Min) mice. Gastroenterology 2010; 138: 1003–1011 e1-5.
Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B . Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 2009; 5: 200–211.
Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA . Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 2009; 137: 1343–1355.
Beebe K, Lee WC, Micchelli CA . JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol 2010; 338: 28–37.
Biteau B, Jasper H . EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development 2011; 138: 1045–1055.
Cordero JB, Stefanatos RK, Myant K, Vidal M, Sansom OJ . Non-autonomous crosstalk between the Jak/Stat and Egfr pathways mediates Apc1-driven intestinal stem cell hyperplasia in the Drosophila adult midgut. Development 2012; 139: 4524–4535.
Karpowicz P, Perez J, Perrimon N . The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development 2010; 137: 4135–4145.
Du XX, Doerschuk CM, Orazi A, Williams DA . A bone marrow stromal-derived growth factor, interleukin-11, stimulates recovery of small intestinal mucosal cells after cytoablative therapy. Blood 1994; 83: 33–37.
Du XX, Williams DA . Interleukin-11: a multifunctional growth factor derived from the hematopoietic microenvironment. Blood 1994; 83: 2023–2030.
Potten CS . Interleukin-11 protects the clonogenic stem cells in murine small-intestinal crypts from impairment of their reproductive capacity by radiation. Int J Cancer 1995; 62: 356–361.
Potten CS . Protection of the small intestinal clonogenic stem cells from radiation-induced damage by pretreatment with interleukin 11 also increases murine survival time. Stem Cells 1996; 14: 452–459.
Orazi A, Du X, Yang Z, Kashai M, Williams DA . Interleukin-11 prevents apoptosis and accelerates recovery of small intestinal mucosa in mice treated with combined chemotherapy and radiation. Lab Invest 1996; 75: 33–42.
Ferrand A, Bertrand C, Portolan G, Cui G, Carlson J, Pradayrol L et al. Signaling pathways associated with colonic mucosa hyperproliferation in mice overexpressing gastrin precursors. Cancer Res 2005; 65: 2770–2777.
Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014; 513: 202–209.
Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA . Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol 2008; 294: R393–R401.
Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 2004; 21: 491–501.
Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009; 15: 103–113.
Seavey MM, Lu LD, Stump KL, Wallace NH, Hockeimer W, O'Kane TM et al. Therapeutic efficacy of CEP-33779, a novel selective JAK2 inhibitor, in a mouse model of colitis-induced colorectal cancer. Mol Cancer Ther 2012; 11: 984–993.
Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 2009; 15: 91–102.
Ernst M, Najdovska M, Grail D, Lundgren-May T, Buchert M, Tye H et al. STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest 2008; 118: 1727–1738.
Judd LM, Menheniott TR, Ling H, Jackson CB, Howlett M, Kalantzis A et al. Inhibition of the JAK2/STAT3 pathway reduces gastric cancer growth in vitro and in vivo. PLoS One 2014; 9: e95993.
Phesse TJ, Buchert M, Stuart E, Flanagan DJ, Faux M, Afshar-Sterle S et al. Partial inhibition of gp130-Jak-Stat3 signaling prevents Wnt-beta-catenin-mediated intestinal tumor growth and regeneration. Sci Signal 2014; 7: ra92.
Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007; 317: 121–124.
Rebouissou S, Amessou M, Couchy G, Poussin K, Imbeaud S, Pilati C et al. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature 2009; 457: 200–204.
Pilati C, Amessou M, Bihl MP, Balabaud C, Nhieu JT, Paradis V et al. Somatic mutations activating STAT3 in human inflammatory hepatocellular adenomas. J Exp Med 2011; 208: 1359–1366.
Poussin K, Pilati C, Couchy G, Calderaro J, Bioulac-Sage P, Bacq Y et al. Biochemical and functional analyses of gp130 mutants unveil JAK1 as a novel therapeutic target in human inflammatory hepatocellular adenoma. Oncoimmunology 2013; 2: e27090.
Fuke H, Shiraki K, Sugimoto K, Tanaka J, Beppu T, Yoneda K et al. Jak inhibitor induces S phase cell-cycle arrest and augments TRAIL-induced apoptosis in human hepatocellular carcinoma cells. Biochem Biophys Res Commun 2007; 363: 738–744.
Mohan CD, Bharathkumar H, Bulusu KC, Pandey V, Rangappa S, Fuchs JE et al. Development of a novel azaspirane that targets the JAK-STAT pathway in hepatocellular carcinoma in vitro and in vivo. J Biol Chem 2014; 289: 34296–34307.
Zhang L, Yang Z, Ma A, Qu Y, Xia S, Xu D et al. Growth arrest and DNA damage 45G down-regulation contributes to Janus kinase/signal transducer and activator of transcription 3 activation and cellular senescence evasion in hepatocellular carcinoma. Hepatology 2014; 59: 178–189.
Grivennikov S, Karin M . Autocrine IL-6 signaling: a key event in tumorigenesis? Cancer Cell 2008; 13: 7–9.
Lederle W, Depner S, Schnur S, Obermueller E, Catone N, Just A et al. IL-6 promotes malignant growth of skin SCCs by regulating a network of autocrine and paracrine cytokines. Int J Cancer 2011; 128: 2803–2814.
Kan Z, Zheng H, Liu X, Li S, Barber TD, Gong Z et al. Whole-genome sequencing identifies recurrent mutations in hepatocellular carcinoma. Genome Res 2013; 23: 1422–1433.
Leibowitz MS, Srivastava RM, Andrade Filho PA, Egloff AM, Wang L, Seethala RR et al. SHP2 is overexpressed and inhibits pSTAT1-mediated APM component expression, T-cell attracting chemokine secretion, and CTL recognition in head and neck cancer cells. Clin Cancer Res 2013; 19: 798–808.
Grandis JR, Drenning SD, Chakraborty A, Zhou MY, Zeng Q, Pitt AS et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth in vitro. J Clin Invest 1998; 102: 1385–1392.
Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S et al. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci USA 2000; 97: 4227–4232.
Yu H, Kortylewski M, Pardoll D . Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 2007; 7: 41–51.
Thompson JE, Cubbon RM, Cummings RT, Wicker LS, Frankshun R, Cunningham BR et al. Photochemical preparation of a pyridone containing tetracycle: a Jak protein kinase inhibitor. Bioorg Med Chem Lett 2002; 12: 1219–1223.
Lucet IS, Fantino E, Styles M, Bamert R, Patel O, Broughton SE et al. The structural basis of Janus kinase 2 inhibition by a potent and specific pan-Janus kinase inhibitor. Blood 2006; 107: 176–183.
Andraos R, Qian Z, Bonenfant D, Rubert J, Vangrevelinghe E, Scheufler C et al. Modulation of activation-loop phosphorylation by JAK inhibitors is binding mode dependent. Cancer Discov 2013; 2: 512–523.
Pardanani A, Laborde RR, Lasho TL, Finke C, Begna K, Al-Kali A et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia 2013; 27: 1322–1327.
Plimack ER, LoRusso PM, McCoon P, Tang W, Krebs AD, Curt G et al. AZD1480: a phase I study of a novel JAK2 inhibitor in solid tumors. Oncologist 2013; 18: 819–820.
van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Garcia Meijide JA, Wagner S et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012; 367: 508–519.
Taylor P, Genovese M, Keystone E, Schlichting D, Beattie S, Macias W . A1.72 Baricitinib, an oral janus kinase inhibitor, in the treatment of rheumatoid arthritis: safety and efficacy in an open-label, long-term extension study1. Ann Rheum Dis 2014; 73: A31.
Norman P . Selective JAK inhibitors in development for rheumatoid arthritis. Expert Opin Investig Drugs 2014; 23: 1067–1077.
Haan C, Rolvering C, Raulf F, Kapp M, Druckes P, Thoma G et al. Jak1 has a dominant role over Jak3 in signal transduction through gammac-containing cytokine receptors. Chem Biol 2011; 18: 314–323.
Thoma G, Druckes P, Zerwes HG . Selective inhibitors of the Janus kinase Jak3—Are they effective? Bioorg Med Chem Lett 2014; 24: 4617–4621.
Kershaw NJ, Murphy JM, Liau NP, Varghese LN, Laktyushin A, Whitlock EL et al. SOCS3 binds specific receptor-JAK complexes to control cytokine signaling by direct kinase inhibition. Nat Struct Mol Biol 2013; 20: 469–476.
Fiskus W, Verstovsek S, Manshouri T, Rao R, Balusu R, Venkannagari S et al. Heat shock protein 90 inhibitor is synergistic with JAK2 inhibitor and overcomes resistance to JAK2-TKI in human myeloproliferative neoplasm cells. Clin Cancer Res 2011; 17: 7347–7358.
Monaghan KA, Khong T, Burns CJ, Spencer A . The novel JAK inhibitor CYT387 suppresses multiple signalling pathways, prevents proliferation and induces apoptosis in phenotypically diverse myeloma cells. Leukemia 2011; 25: 1891–1899.
Derenzini E, Lemoine M, Buglio D, Katayama H, Ji Y, Davis RE et al. The JAK inhibitor AZD1480 regulates proliferation and immunity in Hodgkin lymphoma. Blood Cancer J 2011; 1: e46.
Novotny-Diermayr V, Hart S, Goh KC, Cheong A, Ong LC, Hentze H et al. The oral HDAC inhibitor pracinostat (SB939) is efficacious and synergistic with the JAK2 inhibitor pacritinib (SB1518) in preclinical models of AML. Blood Cancer J 2012; 2: e69.
Wang Y, Fiskus W, Chong DG, Buckley KM, Natarajan K, Rao R et al. Cotreatment with panobinostat and JAK2 inhibitor TG101209 attenuates JAK2V617F levels and signaling and exerts synergistic cytotoxic effects against human myeloproliferative neoplastic cells. Blood 2009; 114: 5024–5033.
Evrot E, Ebel N, Romanet V, Roelli C, Andraos R, Qian Z et al. JAK1/2 and Pan-deacetylase inhibitor combination therapy yields improved efficacy in preclinical mouse models of JAK2V617F-driven disease. Clin Cancer Res 2013; 19: 6230–6241.
Fiskus W, Verstovsek S, Manshouri T, Smith JE, Peth K, Abhyankar S et al. Dual PI3K/AKT/mTOR inhibitor BEZ235 synergistically enhances the activity of JAK2 inhibitor against cultured and primary human myeloproliferative neoplasm cells. Mol Cancer Ther 2013; 12: 577–588.
Pecquet C, Staerk J, Chaligne R, Goss V, Lee KA, Zhang X et al. Induction of myeloproliferative disorder and myelofibrosis by thrombopoietin receptor W515 mutants is mediated by cytosolic tyrosine 112 of the receptor. Blood 2010; 115: 1037–1048.
Choong ML, Pecquet C, Pendharkar V, Diaconu CC, Yong JW, Tai SJ et al. Combination treatment for myeloproliferative neoplasms using JAK and pan-class I PI3K inhibitors. J Cell Mol Med 2013; 17: 1397–1409.
Harir N, Pecquet C, Kerenyi M, Sonneck K, Kovacic B, Nyga R et al. Constitutive activation of Stat5 promotes its cytoplasmic localization and association with PI3-kinase in myeloid leukemias. Blood 2007; 109: 1678–1686.
Will B, Siddiqi T, Jorda MA, Shimamura T, Luptakova K, Staber PB et al. Apoptosis induced by JAK2 inhibition is mediated by Bim and enhanced by the BH3 mimetic ABT-737 in JAK2 mutant human erythroid cells. Blood 2010; 115: 2901–2909.
Joung YH, Na YM, Yoo YB, Darvin P, Sp N, Kang DY et al. Combination of AG490, a Jak2 inhibitor, and methylsulfonylmethane synergistically suppresses bladder tumor growth via the Jak2/STAT3 pathway. Int J Oncol 2014; 44: 883–895.
Durmus S, Xu N, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH . P-glycoprotein (MDR1/ABCB1) and breast cancer resistance protein (BCRP/ABCG2) restrict brain accumulation of the JAK1/2 inhibitor, CYT387. Pharmacol Res 2013; 76: 9–16.
Koppikar P, Bhagwat N, Kilpivaara O, Manshouri T, Adli M, Hricik T et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature 2012; 489: 155–159.
Mencalha AL, Du Rocher B, Salles D, Binato R, Abdelhay E . LLL-3 a STAT3 inhibitor, represses BCR-ABL-positive cell proliferation, activates apoptosis and improves the effects of Imatinib mesylate. Cancer Chemother Pharmacol 2010; 65: 1039–1046.
Wang SJ, Cui HY, Liu YM, Zhao P, Zhang Y, Fu ZG et al. CD147 promotes Src-dependent activation of Rac1 signaling through STAT3/DOCK8 during the motility of hepatocellular carcinoma cells. Oncotarget 2015; 6: 243–257.
Read RD, Bach EA, Cagan RL . Drosophila C-terminal Src kinase negatively regulates organ growth and cell proliferation through inhibition of the Src, Jun N-terminal kinase, and STAT pathways. Mol Cell Biol 2004; 24: 6676–6689.
Marit MR, Chohan M, Matthew N, Huang K, Kuntz DA, Rose DR et al. Random mutagenesis reveals residues of JAK2 critical in evading inhibition by a tyrosine kinase inhibitor. PLoS One 2012; 7: e43437.
Nagai H, Kim YS, Lee KT, Chu MY, Konishi N, Fujimoto J et al. Inactivation of SSI-1, a JAK/STAT inhibitor, in human hepatocellular carcinomas, as revealed by two-dimensional electrophoresis. J Hepatol 2001; 34: 416–421.
Nagai H, Naka T, Terada Y, Komazaki T, Yabe A, Jin E et al. Hypermethylation associated with inactivation of the SOCS-1 gene, a JAK/STAT inhibitor, in human hepatoblastomas. J Hum Genet 2003; 48: 65–69.
Van Schaeybroeck S, Kalimutho M, Dunne PD, Carson R, Allen W, Jithesh PV et al. ADAM17-dependent c-MET-STAT3 signaling mediates resistance to MEK inhibitors in KRAS mutant colorectal cancer. Cell Rep 2014; 7: 1940–1955.
Lee HJ, Zhuang G, Cao Y, Du P, Kim HJ, Settleman J . Drug resistance via feedback activation of stat3 in oncogene-addicted cancer cells. Cancer Cell 2014; 26: 207–221.
Acknowledgements
The work in the authors’ laboratories is supported by the Cancer Council Victoria, grants (#1064987 and #1079257) from the National Health and Medical Research Council, Australia, by Ludwig Cancer Research and by funds from the Operational Infrastructure Support Program provided by the Victorian Government, Australia.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Matthias Ernst and Michael Buchert have filed a patent application on the use of Jak inhibitors for the treatment of colon cancer, and Chris Burns is an inventor on several patents describing Jak inhibitors.
Rights and permissions
About this article
Cite this article
Buchert, M., Burns, C. & Ernst, M. Targeting JAK kinase in solid tumors: emerging opportunities and challenges. Oncogene 35, 939–951 (2016). https://doi.org/10.1038/onc.2015.150
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.150
This article is cited by
-
High-fat diet induced cyclophilin B enhances STAT3/lncRNA-PVT1 feedforward loop and promotes growth and metastasis in colorectal cancer
Cell Death & Disease (2022)
-
STAT3 inhibition suppresses adaptive survival of ALK-rearranged lung cancer cells through transcriptional modulation of apoptosis
npj Precision Oncology (2022)
-
A computationally affordable approach for accurate prediction of the binding affinity of JAK2 inhibitors
Journal of Molecular Modeling (2022)
-
ALKBH5-HOXA10 loop-mediated JAK2 m6A demethylation and cisplatin resistance in epithelial ovarian cancer
Journal of Experimental & Clinical Cancer Research (2021)
-
Targeting STAT3 in Cancer Immunotherapy
Molecular Cancer (2020)