Abstract
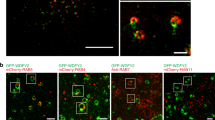
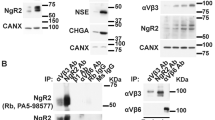
Tissue expression microarrays, employed to determine the players and mechanisms leading to prostate cancer development, have consistently shown that myosin VI, a unique actin-based motor, is upregulated in medium-grade human prostate cancers. Thus, to understand the role of myosin VI in prostate cancer development, we have characterized its intracellular localization and function in the prostate cancer cell line LNCaP. Using light and electron microscopy, we identified myosin VI on Rab5-positive early endosomes, as well as on recycling endosomes and the trans-Golgi network. Intracellular targeting seems to involve two myosin VI-interacting proteins, GIPC and LMTK2, both of which can be co-immunoprecipitated with myosin VI from LNCaP cells. The absence of Disabled-2 (Dab2), a tumour suppressor and myosin VI-binding partner, inhibits recruitment of myosin VI to endocytic structures at the plasma membrane in LNCaP cells, but interestingly has no effect on endocytosis. Small interfering RNA-mediated downregulation of myosin VI expression results in a significant reduction in prostate-specific antigen (PSA) and vascular endothelial growth factor (VEGF) secretion in LNCaP cells. Our results suggest that in prostate cancer cells, myosin VI regulates protein secretion, but the overexpression of myosin VI has no major impact on clathrin-mediated endocytosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ameen N, Apodaca G . (2007). Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic 8: 998–1006.
Ang AL, Taguchi T, Francis S, Folsch H, Murrells LJ, Pypaert M et al. (2004). Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol 167: 531–543.
Aschenbrenner L, Lee T, Hasson T . (2003). Myo6 facilitates the translocation of endocytic vesicles from cell peripheries. Mol Biol Cell 14: 2728–2743.
Au JS, Puri C, Ihrke G, Kendrick-Jones J, Buss F . (2007). Myosin VI is required for sorting of AP-1B-dependent cargo to the basolateral domain in polarized MDCK cells. J Cell Biol 177: 103–114.
Balk SP, Ko YJ, Bubley GJ . (2003). Biology of prostate-specific antigen. J Clin Oncol 21: 383–391.
Borgono CA, Diamandis EP . (2004). The emerging roles of human tissue kallikreins in cancer. Nat Rev Cancer 4: 876–890.
Bunn RC, Jensen MA, Reed BC . (1999). Protein interactions with the glucose transporter binding protein GLUT1CBP that provide a link between GLUT1 and the cytoskeleton. Mol Biol Cell 10: 819–832.
Buss F, Arden SD, Lindsay M, Luzio JP, Kendrick-Jones J . (2001). Myosin VI isoform localized to clathrin-coated vesicles with a role in clathrin-mediated endocytosis. EMBO J 20: 3676–3684.
Buss F, Kendrick-Jones J . (2008). How are the cellular functions of myosin VI regulated within the cell? Biochem Biophys Res Commun 369: 165–175.
Buss F, Kendrick-Jones J, Lionne C, Knight AE, Cote GP, Paul Luzio J . (1998). The localization of myosin VI at the golgi complex and leading edge of fibroblasts and its phosphorylation and recruitment into membrane ruffles of A431 cells after growth factor stimulation. J Cell Biol 143: 1535–1545.
Chibalina MV, Seaman MN, Miller CC, Kendrick-Jones J, Buss F . (2007). Myosin VI and its interacting protein LMTK2 regulate tubule formation and transport to the endocytic recycling compartment. J Cell Sci 120: 4278–4288.
Clements JA, Willemsen NM, Myers SA, Dong Y . (2004). The tissue kallikrein family of serine proteases: functional roles in human disease and potential as clinical biomarkers. Crit Rev Clin Lab Sci 41: 265–312.
Dance AL, Miller M, Seragaki S, Aryal P, White B, Aschenbrenner L et al. (2004). Regulation of myosin-VI targeting to endocytic compartments. Traffic 5: 798–813.
Dunn TA, Chen S, Faith DA, Hicks JL, Platz EA, Chen Y et al. (2006). A novel role of myosin VI in human prostate cancer. Am J Pathol 169: 1843–1854.
Eeles RA, Kote-Jarai Z, Giles GG, Olama AA, Guy M, Jugurnauth SK et al. (2008). Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet 40: 316–321.
Ellis LM, Hicklin DJ . (2008). VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 8: 579–591.
Fazili Z, Sun W, Mittelstaedt S, Cohen C, Xu XX . (1999). Disabled-2 inactivation is an early step in ovarian tumorigenicity. Oncogene 18: 3104–3113.
Geisbrecht ER, Montell DJ . (2002). Myosin VI is required for E-cadherin-mediated border cell migration. Nat Cell Biol 4: 616–620.
Hasson T, Mooseker MS . (1994). Porcine myosin-VI: characterization of a new mammalian unconventional myosin. J Cell Biol 127: 425–440.
Hicklin DJ, Ellis LM . (2005). Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23: 1011–1027.
Kellerman KA, Miller KG . (1992). An unconventional myosin heavy chain gene from Drosophila melanogaster. J Cell Biol 119: 823–834.
Lawrence MG, Veveris-Lowe TL, Whitbread AK, Nicol DL, Clements JA . (2007). Epithelial-mesenchymal transition in prostate cancer and the potential role of kallikrein serine proteases. Cells Tissues Organs 185: 111–115.
Maddugoda MP, Crampton MS, Shewan AM, Yap AS . (2007). Myosin VI and vinculin cooperate during the morphogenesis of cadherin cell cell contacts in mammalian epithelial cells. J Cell Biol 178: 529–540.
Martin DB, Gifford DR, Wright ME, Keller A, Yi E, Goodlett DR et al. (2004). Quantitative proteomic analysis of proteins released by neoplastic prostate epithelium. Cancer Res 64: 347–355.
Morris SM, Arden SD, Roberts RC, Kendrick-Jones J, Cooper JA, Luzio JP et al. (2002). Myosin VI binds to and localises with Dab2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic 3: 331–341.
Morris SM, Cooper JA . (2001). Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic 2: 111–123.
Morriswood B, Ryzhakov G, Puri C, Arden SD, Roberts R, Dendrou C et al. (2007). T6BP and NDP52 are myosin VI binding partners with potential roles in cytokine signalling and cell adhesion. J Cell Sci 120: 2574–2585.
Peden AA, Schonteich E, Chun J, Junutula JR, Scheller RH, Prekeris R . (2004). The RCP-Rab11 complex regulates endocytic protein sorting. Mol Biol Cell 15: 3530–3541.
Puri C, Tosoni D, Comai R, Rabellino A, Segat D, Caneva F et al. (2005). Relationships between EGFR signaling-competent and endocytosis-competent membrane microdomains. Mol Biol Cell 16: 2704–2718.
Rabouille C, Misteli T, Watson R, Warren G . (1995). Reassembly of Golgi stacks from mitotic Golgi fragments in a cell-free system. J Cell Biol 129: 605–618.
Sahlender DA, Roberts RC, Arden SD, Spudich G, Taylor MJ, Luzio JP et al. (2005). Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J Cell Biol 169: 285–295.
Schwahn DJ, Medina D . (1998). p96, a MAPK-related protein, is consistently downregulated during mouse mammary carcinogenesis. Oncogene 17: 1173–1178.
Spudich G, Chibalina MV, Au JS, Arden SD, Buss F, Kendrick-Jones J . (2007). Myosin VI targeting to clathrin-coated structures and dimerization is mediated by binding to Disabled-2 and PtdIns(4,5)P2. Nat Cell Biol 9: 176–183.
Su AI, Welsh JB, Sapinoso LM, Kern SG, Dimitrov P, Lapp H et al. (2001). Molecular classification of human carcinomas by use of gene expression signatures. Cancer Res 61: 7388–7393.
Tseng CP, Ely BD, Li Y, Pong RC, Hsieh JT . (1998). Regulation of rat DOC-2 gene during castration-induced rat ventral prostate degeneration and its growth inhibitory function in human prostatic carcinoma cells. Endocrinology 139: 3542–3553.
Veveris-Lowe TL, Lawrence MG, Collard RL, Bui L, Herington AC, Nicol DL et al. (2005). Kallikrein 4 (hK4) and prostate-specific antigen (PSA) are associated with the loss of E-cadherin and an epithelial-mesenchymal transition (EMT)-like effect in prostate cancer cells. Endocr Relat Cancer 12: 631–643.
Warner CL, Stewart A, Luzio JP, Steel KP, Libby RT, Kendrick-Jones J et al. (2003). Loss of myosin VI reduces secretion and the size of the Golgi in fibroblasts from Snell's waltzer mice. EMBO J 22: 569–579.
Wei S, Dunn TA, Isaacs WB, De Marzo AM, Luo J . (2008). GOLPH2 and MYO6: putative prostate cancer markers localized to the Golgi apparatus. Prostate 68: 1387–1395.
Wells AL, Lin AW, Chen LQ, Safer D, Cain SM, Hasson T et al. (1999). Myosin VI is an actin-based motor that moves backwards. Nature 401: 505–508.
Whitbread AK, Veveris-Lowe TL, Lawrence MG, Nicol DL, Clements JA . (2006). The role of kallikrein-related peptidases in prostate cancer: potential involvement in an epithelial to mesenchymal transition. Biol Chem 387: 707–714.
Yoshida H, Cheng W, Hung J, Montell D, Geisbrecht E, Rosen D et al. (2004). Lessons from border cell migration in the Drosophila ovary: a role for myosin VI in dissemination of human ovarian cancer. Proc Natl Acad Sci USA 101: 8144–8149.
Acknowledgements
We thank A Peden for help with transferrin uptake assays and JP Luzio for helpful discussions. This work was funded by the Cancer Research UK project grant (CP), the Wellcome Trust Senior Fellowship (to FB), the Wellcome Trust PhD studentship (AJK) and was supported by the Medical Research Council. CIMR is in receipt of a strategic award from the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Puri, C., Chibalina, M., Arden, S. et al. Overexpression of myosin VI in prostate cancer cells enhances PSA and VEGF secretion, but has no effect on endocytosis. Oncogene 29, 188–200 (2010). https://doi.org/10.1038/onc.2009.328
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2009.328
Keywords
This article is cited by
-
Myosin VI regulates the spatial organisation of mammalian transcription initiation
Nature Communications (2022)
-
Diverse functions of myosin VI in spermiogenesis
Histochemistry and Cell Biology (2021)
-
Shear Stress Increases V–H\(^{+}\)-ATPase and Acidic Vesicle Number Density, and p-mTORC2 Activation in Prostate Cancer Cells
Cellular and Molecular Bioengineering (2020)
-
NDP52 activates nuclear myosin VI to enhance RNA polymerase II transcription
Nature Communications (2017)
-
miR-143 and miR-145 inhibit gastric cancer cell migration and metastasis by suppressing MYO6
Cell Death & Disease (2017)