Key Points
-
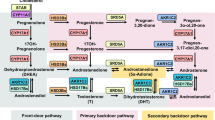
17α-Hydroxylase/C17,20-lyase (CYP17) is a steroidogenic enzyme that is central to the production of androgens, and is targeted by abiraterone in men with castration-resistant prostate cancer
-
Abiraterone is a promiscuous drug, interacting with numerous targets that include CYP11B1 and a panel of hepatic CYP enzymes; these interactions explain the adverse effects profile of the drug
-
Furthermore, inhibition of the 17α-hydroxylase activity of CYP17 is responsible for the secondary mineralocorticoid excess observed in men taking abiraterone; selective C17,20-lyase inhibitors might avoid this effect
-
CYP17 inhibitors that interfere with the androgen receptor (AR) might enhance clinical benefit and might result in increased compliance and reduced risk of drug–drug interactions compared with combined regimens
-
Dual inhibitors of CYP17 and CYP11B1 might improve curative effects in patients with mutated ARs that are agonized by cortisol
-
Dual inhibitors that target the C17,20-lyase activity of CYP17 and CYP11B2 might reduce the risks of cardiovascular complications associated with abiraterone use by mitigating increases in aldosterone levels
Abstract
As the first in class steroid 17α-hydroxylase/C17,20-lyase (CYP17) inhibitor, abiraterone acetate (of which the active metabolite is abiraterone) has been shown to improve overall survival in patients with castration-resistant prostate cancer (CRPC)—in those who are chemotherapy-naive and those previously treated with docetaxel. Furthermore, the clinical success of abiraterone demonstrated that CRPC, which has previously been regarded as an androgen-independent disease, is still driven, at least in part, by androgens. More importantly, abiraterone is a 'promiscuous' drug that interacts with a number of targets, which dictate its clinical benefits and adverse effects profile. Besides CYP17 inhibition, abiraterone acts as an antagonist to the androgen receptor and inhibits 3β-hydroxysteroid dehydrogenase—two effects that potentially contribute to its antitumour effects. However, the inhibition of the 17α-hydroxylase activity of CYP17, CYP11B1 and a panel of hepatic CYP enzymes leads to adverse effects and toxicities that include secondary mineralocorticoid excess. Abiraterone is also associated with increased incidence of cardiac disorders. Under such circumstances, development of new CYP17 inhibitors as an additional line of defence is urgently needed. To achieve enhanced clinical benefits, new strategies are being explored that include selective inhibition of the C17,20-lyase activity of CYP17 and multi-targeting strategies that affect androgen synthesis and signalling at different points. Some of these strategies—including the drugs orteronel, VT-464 and galeterone—are supported by preclinical data and are being explored in the clinic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 62, 10–29 (2012).
van Leeuwen, P. J. et al. Prostate cancer mortality in screen and clinically detected prostate cancer: estimating the screening benefit. Eur. J. Cancer 46, 377–383 (2010).
Siegel, R. et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clin. 62, 220–241 (2012).
Welch, H. G. & Albertsen, P. C. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986–2005. J. Natl Cancer Inst. 101, 1325–1329 (2009).
Holmberg, L. et al. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. N. Engl. J. Med. 347, 781–789 (2002).
Parker, C. Active surveillance: towards a new paradigm in the management of early prostate cancer. Lancet Oncol. 5, 101–106 (2004).
Bannuru, R. R. et al. Comparative evaluation of radiation treatments for clinically localized prostate cancer: an updated systematic review. Ann. Intern. Med. 155, 171–178 (2011).
Meng, M. V. in Current Medical Diagnosis and Treatment 2013 (eds Papadakis, M. A., McPhee, S. J. & Rabow, M. W.) 1630–1638 (The McGraw-Hill Medical, 2013).
Geller, J. Basis for hormonal management of advanced prostate cancer. Cancer 71 (Suppl. 3), 1039–1045 (1993).
Imamoto, T. et al. The role of testosterone in the pathogenesis of prostate cancer. Int. J. Urol. 15, 472–480 (2008).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Nilsson, S. et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 8, 587–589 (2007).
Fizazi, K. et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377, 813–822 (2011).
Yin, L., Hu, Q. & Hartmann, R. W. Recent progress in pharmaceutical therapies for castration-resistant prostate cancer. Int. J. Mol. Sci. 14, 13958–13978 (2013).
Franchimont, P. Regulation of gonadal androgen secretion. Horm. Res. 18, 7–17 (1983).
Rainey, W. E. & Nakamura, Y. Regulation of the adrenal androgen biosynthesis. J. Steroid. Biochem. Mol. Biol. 108, 281–286 (2008).
Bolton, E. C. et al. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 21, 2005–2017 (2007).
Zhu, M. L. & Kyprianou, N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr. Relat. Cancer 15, 841–849 (2008).
Migliaccio, A. et al. Steroid-induced androgen receptor-oestradiol receptor β-Src complex triggers prostate cancer cell proliferation. EMBO J. 19, 5406–5417 (2000).
Titus, M. A., Schell, M. J., Lih, F. B., Tomer, K. B. & Mohler, J. L. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin. Cancer Res. 11, 4653–4657 (2005).
Suzuki, H. et al. Codon 877 mutation in the androgen receptor gene in advanced prostate cancer: relation to antiandrogen withdrawal syndrome. Prostate 29, 153–158 (1996).
Hara, T. et al. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res. 63, 149–153 (2003).
Zhao, X. Y. et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat. Med. 6, 703–706 (2000).
Cai, C. et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 71, 6503–6513 (2011).
Montgomery, R. B. et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 68, 4447–4454 (2008).
Chang, K. H. et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc. Natl Acad. Sci. USA 108, 13728–13733 (2011).
Mohler, J. L., Titus, M. A. & Wilson, E. M. Potential prostate cancer drug target: bioactivation of androstanediol by conversion to dihydrotestosterone. Clin. Cancer Res. 17, 5844–5849 (2011).
Small, E. J., Baron, A. D., Fippin, L. & Apodaca, D. Ketoconazole retains activity in advanced prostate cancer patients with progression despite flutamide withdrawal. J. Urol. 157, 1204–1207 (1997).
Attard, G. et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol. 26, 4563–4571 (2008).
DeVore, N. M. & Scott, E. E. Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001. Nature 482, 116–120 (2012).
Hu, Q., Negri, M., Olgen, S. & Hartmann, R. W. The role of fluorine substitution in biphenyl methylene imidazole type CYP17 inhibitors for the treatment of prostate carcinoma. Chem. Med. Chem. 5, 899–910 (2010).
Hu, Q., Yin, L., Jagusch, C., Hille, U. E. & Hartmann, R. W. Isopropylidene substitution increases activity and selectivity of biphenyl methylene 4-pyridine type CYP17 inhibitors. J. Med. Chem. 53, 5049–5053 (2010).
Hu, Q. et al. Synthesis, biological evaluation, and molecular modeling studies of methylene imidazole substituted biaryls as inhibitors of human 17α-hydroxylase-17,20-lyase (CYP17)—part II: core rigidification and influence of substituents at the methylene bridge. Bioorg. Med. Chem. 16, 7715–7727 (2008).
Hille, U. E. et al. Novel CYP17 inhibitors: synthesis, biological evaluation, structure-activity relationships and modelling of methoxy- and hydroxy-substituted methyleneimidazolyl biphenyls. Eur. J. Med. Chem. 44, 2765–2775 (2009).
Pinto-Bazurco Mendieta, M. A. et al. CYP17 inhibitors. Annulations of additional rings in methylene imidazole substituted biphenyls: synthesis, biological evaluation and molecular modelling. Arch. Pharm. (Weinheim) 341, 597–609 (2008).
Jagusch, C. et al. Synthesis, biological evaluation and molecular modelling studies of methyleneimidazole substituted biaryls as inhibitors of human 17α-hydroxylase-17,20-lyase (CYP17). Part I: heterocyclic modifications of the core structure. Bioorg. Med. Chem. 16, 1992–2010 (2008).
Abadi, A. H., Abou-Seri, S. M., Hu, Q., Negri, M. & Hartmann, R. W. Synthesis and biological evaluation of imidazolylmethylacridones as cytochrome P-450 enzymes inhibitors. Med. Chem. Comm. 3, 663–666 (2012).
Hille, U. E. et al. Steroidogenic cytochrome P450 (CYP) enzymes as drug targets: combining substructures of known CYP inhibitors leads to compounds with different inhibitory profile. C. R. Chim. 12, 1117–1126 (2009).
Yin, L. & Hu, Q. Drug discovery for breast cancer and coinstantaneous cardiovascular disease: what is the future? Future Med. Chem. 5, 359–362 (2013).
Yin, L. et al. Novel imidazol-1-ylmethyl substituted 1,2,5,6-tetrahydropyrrolo[3,2,1-ij]quinolin-4-ones as potent and selective CYP11B1 inhibitors for the treatment of Cushing's syndrome. J. Med. Chem. 55, 6629–6633 (2012).
Emmerich, J., Hu, Q., Hanke, N. & Hartmann, R. W. Cushing's syndrome: development of highly potent and selective CYP11B1 inhibitors of the (pyridylmethyl)pyridine type. J. Med. Chem. 56, 6022–6032 (2013).
Gobbi, S. et al. Modulation of cytochromes P450 with xanthone-based molecules: from aromatase to aldosterone synthase and steroid 1β-hydroxylase inhibition. J. Med. Chem. 56, 1723–1729 (2013).
Yin, L., Hu, Q. & Hartmann, R. W. 3-Pyridinyl substituted aliphatic cycles as CYP11B2 inhibitors: aromaticity abolishment of the core significantly increased selectivity over CYP1A2. PLoS ONE 7, e48048 (2012).
Hu, Q., Yin, L. & Hartmann, R. W. Selective dual inhibitors of CYP19 and CYP11B2: targeting cardiovascular diseases hiding in the shadow of breast cancer. J. Med. Chem. 55, 7080–7089 (2012).
Yin, L., Hu, Q. & Hartmann, R. W. Tetrahydropyrroloquinolinone type dual inhibitors of aromatase/aldosterone synthase as a novel strategy for breast cancer patients with elevated cardiovascular risks. J. Med. Chem. 56, 460–470 (2013).
Jarman, M., Barrie, S. E. & Llera, J. M. The 16,17-double bond is needed for irreversible inhibition of human cytochrome P45017α by abiraterone (17-(3-pyridyl)androsta-5,16-dien-3β-ol) and related steroidal inhibitors. J. Med. Chem. 41, 5375–5381 (1998).
Janseen. Highlights of prescribing information: Zytiga®[online], (2013).
Ryan, C. J. et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J. Clin. Oncol. 28, 1481–1488 (2010).
Acharya, M. et al. A phase I, open-label, single-dose, mass balance study of 14C-labeled abiraterone acetate in healthy male subjects. Xenobiotica 43, 379–389 (2013).
O'Donnell, A. et al. Hormonal impact of the 17α-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br. J. Cancer 90, 2317–2325 (2004).
Tannock, I. et al. Treatment of metastatic prostatic cancer with low-dose prednisone: evaluation of pain and quality of life as pragmatic indices of response. J. Clin. Oncol. 7, 590–597 (1989).
Attard, G. et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J. Clin. Oncol. 27, 3742–3748 (2009).
Ryan, C. J. et al. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin. Cancer Res. 17, 4854–4861 (2011).
Danila, D. C. et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J. Clin. Oncol. 28, 1496–1501 (2010).
Reid, A. H. et al. Significant and sustained antitumor activity in postdocetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J. Clin. Oncol. 28, 1489–1495 (2010).
Ryan, C. J. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 368, 138–148 (2013).
Logothetis, C. J. et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol. 13, 1210–1217 (2012).
Sternberg, C. N. et al. Effect of abiraterone acetate on fatigue in patients with metastatic castration-resistant prostate cancer after docetaxel chemotherapy. Ann. Oncol. 24, 1017–1025 (2013).
Efstathiou, E. et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J. Clin. Oncol. 30, 637–643 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011).
Fizazi, K. et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 13, 983–992 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Small, E. J. et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J. Clin. Oncol. 22, 1025–1033 (2004).
Eisner, J. R. et al. Assessment of steroid hormones upstream of P450c17 (CYP17) in chemically castrate male rhesus monkeys following treatment with the CYP17 inhibitors VT-464 and abiraterone acetate (AA) [abstract]. Endocr. Rev. 33 (03_MeetingAbstracts), SAT-266 (2012).
Potter, G. A., Barrie, S. E., Jarman, M. & Rowlands, M. G. Novel steroidal inhibitors of human cytochrome P450 17α-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J. Med. Chem. 38, 2463–2471 (1995).
Li, R. et al. Abiraterone inhibits 3β-hydroxysteroid dehydrogenase: a rationale for increasing drug exposure in castration-resistant prostate cancer. Clin. Cancer Res. 18, 3571–3579 (2012).
Soifer, H. S. et al. Direct regulation of androgen receptor activity by potent CYP17 inhibitors in prostate cancer cells. J. Biol. Chem. 287, 3777–3787 (2012).
Yamaoka, M. et al. Orteronel (TAK-700), a novel non-steroidal 17,20-lyase inhibitor: effects on steroid synthesis in human and monkey adrenal cells and serum steroid levels in cynomolgus monkeys. J. Steroid Biochem. Mol. Biol. 129, 115–128 (2012).
Kaku, T. et al. Discovery of orteronel (TAK-700), a naphthylmethylimidazole derivative, as a highly selective 17,20-lyase inhibitor with potential utility in the treatment of prostate cancer. Bioorg. Med. Chem. 19, 6383–6399 (2011).
Dreicer, R. et al. Safety, pharmacokinetics, and efficacy of TAK-700 in metastatic castration-resistant prostate cancer: a phase I/II, open-label study [abstract 3084]. J. Clin. Oncol. 28 (Suppl. 15), a3084 (2010).
Agus, D. B. et al. Safety, efficacy, and pharmacodynamics of the investigational agent orteronel (TAK-700) in metastatic castration-resistant prostate cancer (mCRPC): updated data from a phase I/II study [abstract 98]. J. Clin. Oncol. 30 (Suppl. 5), a98 (2012).
George, D. J. et al. Safety and activity of the investigational agent orteronel (ortl) without prednisone in men with nonmetastatic castration-resistant prostate cancer (nmCRPC) and rising prostate-specific antigen (PSA): updated results of a phase II study [abstract 4549]. J. Clin. Oncol. 30 (Suppl. 5), a4549 (2012).
Dreicer, R. et al. A phase III, randomized, double-blind, multicenter trial comparing the investigational agent orteronel (TAK-700) plus prednisone (P) with placebo plus P in patients with metastatic castration-resistant prostate cancer (mCRPC) that has progressed during or following docetaxel-based therapy [abstract TPS4693]. J. Clin. Oncol. 30 (Suppl. 15), aTPS4693 (2012).
Eisner, J. R. et al. VT-464: a novel, selective inhibitor of P450c17(CYP17)-17,20 lyase for castration-refractory prostate cancer (CRPC) [abstract 198]. J. Clin. Oncol. 30 (Suppl. 5), a198 (2012).
Abbott, D. H. et al. Plasma steroid concentrations in male rhesus monkeys following treatment with the P450c17 (CYP17) inhibitors VT-464 and abiraterone acetate: a comparison to human 17,20-Lyase (lyase) and combined lyase/17α-hydroxylase (hydroxylase) deficiencies [abstract]. Endocr. Rev. 33 (03_MeetingAbstracts), SAT-256 (2012).
Pisle, S. T. et al. Activity of VT-464, a selective CYP17 lyase inhibitor, in the LNCaP prostate cancer xenograft model [abstract 64]. J. Clin. Oncol. 30 (Suppl. 5), a64 (2012).
European Medicines Agency. ClinicalTrialsRegister.eu [online], (2013).
Mostaghel, E. A. et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin. Cancer Res. 17, 5913–5925 (2011).
Handratta, V. D. et al. Novel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft model. J. Med. Chem. 48, 2972–2984 (2005).
Vasaitis, T. et al. Androgen receptor inactivation contributes to antitumor efficacy of CYP17 inhibitor VN/124-1 in prostate cancer. Mol. Cancer Ther. 7, 2348–2357 (2008).
Bruno, R., Gover, T., Burger, A., Brodie, A. M. H. & Njar, V. C. O. 17α-Hydroxylase/17,20 lyase inhibitor VN/124–1 inhibits growth of androgen independent prostate cancer cells via induction of the endoplasmic reticulum stress response. Mol. Cancer Ther. 7, 2828–2836 (2008).
Bruno, R. D. et al. Synthesis and biological evaluations of putative metabolically stable analogs of VN/124-1 (TOK-001): head to head anti-tumor efficacy evaluation of VN/124-1 (TOK-001) and abiraterone in LAPC-4 human prostate cancer xenograft model. Steroids 76, 1268–1279 (2011).
American Association for Cancer Research. Early clinical data show galeterone safe, effective against prostate cancer [online], (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Dutt, S. S. & Gao, A. C. Molecular mechanisms of castration-resistant prostate cancer progression. Future Oncol. 5, 1403–1413 (2009).
Hu, Q., Jagusch, C., Hille, U. E., Haupenthal, J. & Hartmann, R. W. Replacement of imidazolyl by pyridyl in biphenylmethylenes results in selective CYP17 and dual CYP17/CYP11B1 inhibitors for the treatment of prostate cancer. J. Med. Chem. 53, 5749–5758 (2010).
Pinto-Bazurco Mendieta, M. A., Hu, Q., Engel, M. & Hartmann, R. W. Highly potent and selective non-steroidal dual inhibitors of CYP17/CYP11B2 for the treatment of prostate cancer to reduce risks of cardiovascular diseases. J. Med. Chem. 56, 6101–6107 (2013).
Efstathiou, J. A. et al. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31. J. Clin. Oncol. 27, 92–99 (2008).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Hu, Q. & Hartmann, R. W. in Cancer Drug Design and Discovery (ed. Neidle, S.) 319–356 (Academic Press, 2014).
Acknowledgements
The authors would like to express their gratitude to Prof. Dr Rolf W. Hartmann for leading them into the field of steroidogenic CYP enzymes.
Author information
Authors and Affiliations
Contributions
Q. Hu researched the data for the article. Both authors discussed the article's content, wrote the manuscript and edited it before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Yin, L., Hu, Q. CYP17 inhibitors—abiraterone, C17,20-lyase inhibitors and multi-targeting agents. Nat Rev Urol 11, 32–42 (2014). https://doi.org/10.1038/nrurol.2013.274
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2013.274
This article is cited by
-
Selective steroidogenic cytochrome P450 haem iron ligation by steroid-derived isonitriles
Communications Chemistry (2023)
-
Difluoroborate complexes in the synthesis of heterocyclic (13α)-estrone derivatives modified at the A-ring
Russian Chemical Bulletin (2023)
-
Synthesis of steroids containing N’-alkoxydiazene N-oxide groups
Russian Chemical Bulletin (2022)
-
Corticosteroid switch from prednisone to dexamethasone in metastatic castration-resistant prostate cancer patients with biochemical progression on abiraterone acetate plus prednisone
BMC Cancer (2021)
-
Prognostic role of the duration of response to androgen deprivation therapy in patients with metastatic castration resistant prostate cancer treated with enzalutamide or abiraterone acetate
Prostate Cancer and Prostatic Diseases (2021)