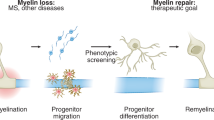
Abstract
The myelin sheath wraps large axons in both the CNS and the PNS, and is a key determinant of efficient axonal function and health. Myelin is targeted in a series of diseases, notably multiple sclerosis (MS). In MS, demyelination is associated with progressive axonal damage, which determines the level of patient disability. The few treatments that are available for combating myelin damage in MS and related disorders, which largely comprise anti-inflammatory drugs, only show limited efficacy in subsets of patients. More-effective treatment of myelin disorders will probably be accomplished by early intervention with combinatorial therapies that target inflammation and other processes—for example, signaling pathways that promote remyelination. Indeed, evidence suggests that such pathways might be impaired in pathology and, hence, contribute to the failure of remyelination in such diseases. In this article, we review the molecular basis of signaling pathways that regulate myelination in the CNS and PNS, with a focus on signals that affect differentiation of myelinating glia. We also discuss factors such as extracellular molecules that act as modulators of these pathways. Finally, we consider the few preclinical and clinical trials of agents that augment this signaling.
Key Points
-
Myelinating glia and their associated axons transmit reciprocal signals that are necessary for the development and maintenance of the myelin–axon unit
-
Both extracellular and intracellular components of myelin–axon signaling pathways are perturbed in myelin diseases, thereby causing axonal damage
-
The level of disability in patients with myelin disorders correlates more with the extent of axonal damage than with the degree of myelin alteration
-
Evidence suggests that insults to myelin or myelinating glia cause secondary axonal damage; thus, myelin or glia are logical targets for early therapeutic intervention
-
Preclinical trials of agents that promote myelination provide hope that combinatorial treatments that target both this process and inflammation can be developed for myelin-related diseases
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Salzer, J. L., Brophy, P. J. & Peles, E. Molecular domains of myelinated axons in the peripheral nervous system. Glia 56, 1532–1540 (2008).
Nave, K. A. & Trapp, B. D. Axon–glial signaling and the glial support of axon function. Annu. Rev. Neurosci. 31, 535–561 (2008).
Bozzali, M. & Wrabetz, L. Axonal signals and oligodendrocyte differentiation. Neurochem. Res. 29, 979–988 (2004).
Simons, M. & Trajkovic, K. Neuron–glia communication in the control of oligodendrocyte function and myelin biogenesis. J. Cell Sci. 119, 4381–4389 (2006).
Woodhoo, A. & Sommer, L. Development of the Schwann cell lineage: from the neural crest to the myelinated nerve. Glia 56, 1481–1490 (2008).
Jessen, K. R. & Mirsky, R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia 56, 1552–1565 (2008).
Lazzarini, R. A. (Ed.) Myelin Biology and Disorders (Elselvier Academic Press, San Diego, 2004).
Trapp, B. D. & Nave, K. A. Multiple sclerosis: an immune or neurodegenerative disorder? Annu. Rev. Neurosci. 31, 247–269 (2008).
Scherer, S. S. & Wrabetz, L. Molecular mechanisms of inherited demyelinating neuropathies. Glia 56, 1578–1589 (2008).
Galbiati, F. et al. Combined hematopoietic and lentiviral gene-transfer therapies in newborn Twitcher mice reveal contemporaneous neurodegeneration and demyelination in Krabbe disease. J. Neurosci. Res. 87, 1748–1759 (2009).
Murinson, B. B., Archer, D. R., Li, Y. & Griffin, J. W. Degeneration of myelinated efferent fibers prompts mitosis in Remak Schwann cells of uninjured C-fiber afferents. J. Neurosci. 25, 1179–1187 (2005).
Lopez-Diego, R. S. & Weiner, H. L. Novel therapeutic strategies for multiple sclerosis—a multifaceted adversary. Nat. Rev. Drug Discov. 7, 909–925 (2008).
Franklin, R. J. & ffrench-Constant, C. Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 9, 839–855 (2008).
Langley, J. N. & Anderson, H. K. On the union of the fifth cervical nerve with the superior cervical ganglion. J. Physiol. 30, 439–442 (1904).
Weinberg, H. J. & Spencer, P. S. Studies on the control of myelinogenesis. II. Evidence for neuronal regulation of myelin production. Brain Res. 113, 363–378 (1976).
Aguayo, A. J., Epps, J., Charron, L. & Bray, G. M. Multipotentiality of Schwann cells in cross-anastomosed and grafted myelinated and unmyelinated nerves: quantitative microscopy and radioautography. Brain Res. 104, 1–20 (1976).
Lubetzki, C. et al. Even in culture, oligodendrocytes myelinate solely axons. Proc. Natl Acad. Sci. USA 90, 6820–6824 (1993).
Duncan, D. A relation between axon diameter and myelination determined by measurement of myelinated spinal root fibers. J. Comp. Neurol. 60, 437–471 (1934).
Voyvodic, J. T. Target size regulates calibre and myelination of sympathetic axons. Nature 342, 430–433 (1989).
Friede, R. L. & Samorajski, T. Relation between the number of myelin lamellae and axon circumference in fibers of vagus and sciatic nerves of mice. J. Comp. Neurol. 130, 223–231 (1967).
Gyllensten, L. & Malmfors, T. Myelinization of the optic nerve and its dependence on visual function—a quantitative investigation in mice. J. Embryol. Exp. Morphol. 11, 255–266 (1963).
Tauber, H., Waehneldt, T. V. & Neuhoff, V. Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neurosci. Lett. 16, 235–238 (1980).
Stevens, B., Porta, S., Haak, L. L., Gallo, V. & Fields, R. D. Adenosine: a neuron–glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36, 855–868 (2002).
Stevens, B., Tanner, S. & Fields, R. D. Control of myelination by specific patterns of neural impulses. J. Neurosci. 18, 9303–9311 (1998).
Demerens, C. et al. Induction of myelination in the central nervous system by electrical activity. Proc. Natl Acad. Sci. USA 93, 9887–9892 (1996).
Birchmeier, C. & Nave, K. A. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia 56, 1491–1497 (2008).
Falls, D. L. Neuregulins and the neuromuscular system: 10 years of answers and questions. J. Neurocytol. 32, 619–647 (2003).
Mei, L. & Xiong, W. C. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 9, 437–452 (2008).
Michailov, G. V. et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science 304, 700–703 (2004).
Taveggia, C. et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 47, 681–694 (2005).
Vartanian, T., Fischbach, G. & Miller, R. Failure of spinal cord oligodendrocyte development in mice lacking neuregulin. Proc. Natl Acad. Sci. USA 96, 731–745 (1999).
Sussman, C. R., Dyer, K. L., Marchionni, M. & Miller, R. H. Local control of oligodendrocyte development in isolated dorsal mouse spinal cord. J. Neurosci. Res. 59, 413–420 (2000).
Sussman, C. R., Vartanian, T. & Miller, R. H. The ErbB4 neuregulin receptor mediates suppression of oligodendrocyte maturation. J. Neurosci. 25, 5757–5762 (2005).
Roy, K. et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc. Natl Acad. Sci. USA 104, 8131–8136 (2007).
Taveggia, C. et al. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia 56, 284–293 (2008).
Brinkmann, B. G. et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron 59, 581–595 (2008).
Fruttiger, M. et al. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development 126, 457–467 (1999).
Chun, S. J., Rasband, M. N., Sidman, R. L., Habib, A. A. & Vartanian, T. Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination. J. Cell Biol. 163, 397–408 (2003).
Sperber, B. R. et al. A unique role for Fyn in CNS myelination. J. Neurosci. 21, 2039–2047 (2001).
Kim, H. J. et al. WAVE1 is required for oligodendrocyte morphogenesis and normal CNS myelination. J. Neurosci. 26, 5849–5859 (2006).
Atanasoski, S. et al. ErbB2 signaling in Schwann cells is mostly dispensable for maintenance of myelinated peripheral nerves and proliferation of adult Schwann cells after injury. J. Neurosci. 26, 2124–2131 (2006).
Zanazzi, G. et al. Glial growth factor/neuregulin inhibits Schwann cell myelination and induces demyelination. J. Cell Biol. 152, 1289–1299 (2001).
Guertin, A. D., Zhang, D. P., Mak, K. S., Alberta, J. A. & Kim, H. A. Microanatomy of axon/glial signaling during Wallerian degeneration. J. Neurosci. 25, 3478–3487 (2005).
Aguirre, A., Dupree, J. L., Mangin, J. M. & Gallo, V. A functional role for EGFR signaling in myelination and remyelination. Nat. Neurosci. 10, 990–1002 (2007).
Yang, P., Baker, K. A. & Hagg, T. The ADAMs family: coordinators of nervous system development, plasticity and repair. Prog. Neurobiol. 79, 73–94 (2006).
Sagane, K. et al. Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice. BMC Neurosci. 6, 33 (2005).
Sagane, K., Ishihama, Y. & Sugimoto, H. LGI1 and LGI4 bind to ADAM22, ADAM23 and ADAM11. Int. J. Biol. Sci. 4, 387–396 (2008).
Bermingham, J. R. Jr et al. The claw paw mutation reveals a role for Lgi4 in peripheral nerve development. Nat. Neurosci. 9, 76–84 (2006).
Wakatsuki, S., Yumoto, N., Komatsu, K., Araki, T. & Sehara-Fujisawa, A. Roles of meltrin-β/ADAM19 in progression of Schwann cell differentiation and myelination during sciatic nerve regeneration. J. Biol. Chem. 284, 2957–2966 (2009).
Ohno, M. et al. Nardilysin regulates axonal maturation and myelination in the central and peripheral nervous system. Nat. Neurosci. 12, 1506–1513 (2009).
Willem, M. et al. Control of peripheral nerve myelination by the β-secretase BACE1. Science 314, 664–666 (2006).
Hu, X. et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci. 9, 1520–1525 (2006).
Hu, X. et al. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 22, 2970–2980 (2008).
Watkins, T. A., Emery, B., Mulinyawe, S. & Barres, B. A. Distinct stages of myelination regulated by γ-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron 60, 555–569 (2008).
Bao, J. et al. Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat. Neurosci. 7, 1250–1258 (2004).
Bao, J., Wolpowitz, D., Role, L. W. & Talmage, D. A. Back signaling by the Nrg-1 intracellular domain. J. Cell Biol. 161, 1133–1141 (2003).
Zampieri, N., Xu, C. F., Neubert, T. A. & Chao, M. V. Cleavage of p75 neurotrophin receptor by α-secretase and γ-secretase requires specific receptor domains. J. Biol. Chem. 280, 14563–14571 (2005).
Fortini, M. E. γ-Secretase-mediated proteolysis in cell-surface-receptor signalling. Nat. Rev. Mol. Cell Biol. 3, 673–684 (2002).
Carson, M. J., Behringer, R. R., Brinster, R. L. & McMorris, F. A. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron 10, 729–740 (1993).
Ness, J. K., Mitchell, N. E. & Wood, T. L. IGF-I and NT-3 signaling pathways in developing oligodendrocytes: differential regulation and activation of receptors and the downstream effector Akt. Dev. Neurosci. 24, 437–445 (2002).
Swamydas, M., Bessert, D. & Skoff, R. Sexual dimorphism of oligodendrocytes is mediated by differential regulation of signaling pathways. J. Neurosci. Res. 87, 3306–3319 (2009).
Flores, A. I. et al. Constitutively active Akt induces enhanced myelination in the CNS. J. Neurosci. 28, 7174–7183 (2008).
Narayanan, S. P., Flores, A. I., Wang, F. & Macklin, W. B. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J. Neurosci. 29, 6860–6870 (2009).
Tyler, W. A. et al. Activation of the mammalian target of rapamycin (mTOR) is essential for oligodendrocyte differentiation. J. Neurosci. 29, 6367–6378 (2009).
Wegner, M. A matter of identity: transcriptional control in oligodendrocytes. J. Mol. Neurosci. 35, 3–12 (2008).
Emery, B. et al. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell 138, 172–185 (2009).
Howng, S. Y. et al. ZFP191 is required by oligodendrocytes for CNS myelination. Genes Dev. 24, 301–311 (2010).
Svaren, J. & Meijer, D. The molecular machinery of myelin gene transcription in Schwann cells. Glia 56, 1541–1551 (2008).
Mager, G. M. et al. Active gene repression by the Egr2.NAB complex during peripheral nerve myelination. J. Biol. Chem. 283, 18187–18197 (2008).
Parkinson, D. B. et al. c-Jun is a negative regulator of myelination. J. Cell Biol. 181, 625–637 (2008).
Woodhoo, A. et al. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nat. Neurosci. 12, 839–847 (2009).
Giambonini-Brugnoli, G., Buchstaller, J., Sommer, L., Suter, U. & Mantei, N. Distinct disease mechanisms in peripheral neuropathies due to altered peripheral myelin protein 22 gene dosage or a Pmp22 point mutation. Neurobiol. Dis. 18, 656–668 (2005).
Kao, S. C. et al. Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell differentiation. Science 323, 651–654 (2009).
Graef, I. A. et al. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell 113, 657–670 (2003).
Maurel, P. et al. Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination. J. Cell Biol. 178, 861–874 (2007).
Spiegel, I. et al. A central role for Necl4 (SynCAM4) in Schwann cell–axon interaction and myelination. Nat. Neurosci. 10, 861–869 (2007).
Ogita, H. & Takai, Y. Nectins and nectin-like molecules: roles in cell adhesion, polarization, movement, and proliferation. IUBMB Life 58, 334–343 (2006).
Chan, J. R. et al. The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science 314, 832–836 (2006).
Park, J. et al. Disruption of Nectin-like 1 cell adhesion molecule leads to delayed axonal myelination in the CNS. J. Neurosci. 28, 12815–12819 (2008).
Pellissier, F., Gerber, A., Bauer, C., Ballivet, M. & Ossipow, V. The adhesion molecule Necl-3/SynCAM-2 localizes to myelinated axons, binds to oligodendrocytes and promotes cell adhesion. BMC Neurosci. 8, 90 (2007).
D'Souza, B., Miyamoto, A. & Weinmaster, G. The many facets of Notch ligands. Oncogene 27, 5148–5167 (2008).
John, G. R. et al. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat. Med. 8, 1115–1121 (2002).
Wang, S. et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 21, 63–75 (1998).
Genoud, S. et al. Notch1 control of oligodendrocyte differentiation in the spinal cord. J. Cell Biol. 158, 709–718 (2002).
Givogri, M. I. et al. Central nervous system myelination in mice with deficient expression of Notch1 receptor. J. Neurosci. Res. 67, 309–320 (2002).
Zhang, Y. et al. Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proc. Natl Acad. Sci. USA 106, 19162–19167 (2009).
Nakahara, J., Kanekura, K., Nawa, M., Aiso, S. & Suzuki, N. Abnormal expression of TIP30 and arrested nucleocytoplasmic transport within oligodendrocyte precursor cells in multiple sclerosis. J. Clin. Invest. 119, 169–181 (2009).
Stidworthy, M. F. et al. Notch1 and Jagged1 are expressed after CNS demyelination, but are not a major rate-determining factor during remyelination. Brain 127, 1928–1941 (2004).
Hu, Q. D. et al. F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell 115, 163–175 (2003).
Morrison, S. J. et al. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 101, 499–510 (2000).
Parkinson, D. B. et al. Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J. Cell Biol. 164, 385–394 (2004).
Shen, S. et al. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat. Neurosci. 11, 1024–1034 (2008).
Camelo, S. et al. Transcriptional therapy with the histone deacetylase inhibitor trichostatin A ameliorates experimental autoimmune encephalomyelitis. J. Neuroimmunol. 164, 10–21 (2005).
Kim, J. Y. et al. HDAC1 nuclear export induced by pathological conditions is essential for the onset of axonal damage. Nat. Neurosci. 13, 180–189 (2010).
Lonigro, A. & Devaux, J. J. Disruption of neurofascin and gliomedin at nodes of Ranvier precedes demyelination in experimental allergic neuritis. Brain 132, 260–273 (2009).
Howell, O. W. et al. Disruption of neurofascin localization reveals early changes preceding demyelination and remyelination in multiple sclerosis. Brain 129, 3173–3185 (2006).
Wolswijk, G. & Balesar, R. Changes in the expression and localization of the paranodal protein Caspr on axons in chronic multiple sclerosis. Brain 126, 1638–1649 (2003).
Coman, I. et al. Nodal, paranodal and juxtaparanodal axonal proteins during demyelination and remyelination in multiple sclerosis. Brain 129, 3186–3195 (2006).
Arroyo, E. J. et al. Genetic dysmyelination alters the molecular architecture of the nodal region. J. Neurosci. 22, 1726–1737 (2002).
Susuki, K. et al. Anti-GM1 antibodies cause complement-mediated disruption of sodium channel clusters in peripheral motor nerve fibers. J. Neurosci. 27, 3956–3967 (2007).
Mathey, E. K. et al. Neurofascin as a novel target for autoantibody-mediated axonal injury. J. Exp. Med. 204, 2363–2372 (2007).
Eldridge, C. F., Bunge, M. B. & Bunge, R. P. Differentiation of axon-related Schwann cell in vitro: II. Control of myelin formation by basal lamina. J. Neurosci. 9, 625–638 (1989).
Eldridge, C. F., Bunge, M. B., Bunge, R. P. & Wood, P. M. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J. Cell Biol. 105, 1023–1034 (1987).
Yu, W. M., Feltri, M. L., Wrabetz, L., Strickland, S. & Chen, Z. L. Schwann cell-specific ablation of laminin γ1 causes apoptosis and prevents proliferation. J. Neurosci. 25, 4463–4472 (2005).
Yang, D. et al. Coordinate control of axon defasciculation and myelination by laminin-2 and -8. J. Cell Biol. 168, 655–666 (2005).
Colognato, H. et al. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat. Cell Biol. 4, 833–841 (2002).
Colognato, H., Ramachandrappa, S., Olsen, I. M. & ffrench-Constant, C. Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J. Cell Biol. 167, 365–375 (2004).
Kramer, E. M., Klein, C., Koch, T., Boytinck, M. & Trotter, J. Compartmentation of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J. Biol. Chem. 274, 29042–29049 (1999).
Relucio, J., Tzvetanova, I. D., Ao, W., Lindquist, S. & Colognato, H. Laminin alters fyn regulatory mechanisms and promotes oligodendrocyte development. J. Neurosci. 29, 11794–11806 (2009).
Laursen, L. S., Chan, C. W. & ffrench-Constant, C. An integrin–contactin complex regulates CNS myelination by differential Fyn phosphorylation. J. Neurosci. 29, 9174–9185 (2009).
Haines, J. D., Fragoso, G., Hossain, S., Mushynski, W. E. & Almazan, G. p38 Mitogen-activated protein kinase regulates myelination. J. Mol. Neurosci. 35, 23–33 (2008).
Camara, J. et al. Integrin-mediated axoglial interactions initiate myelination in the central nervous system. J. Cell Biol. 185, 699–712 (2009).
Barros, C. S. et al. β1 integrins are required for normal CNS myelination and promote AKT-dependent myelin outgrowth. Development 136, 2717–2724 (2009).
Benninger, Y. et al. β1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination. J. Neurosci. 26, 7665–7673 (2006).
Jaros, E. & Jenkison, M. Quantitative studies of the abnormal axon-Schwann cell relationship in the peripheral motor and sensory nerves of the dystrophic mouse. Brain Res. 258, 181–196 (1983).
Saito, F. et al. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron 38, 747–758 (2003).
Nodari, A. et al. α6β4 integrin and dystroglycan cooperate to stabilize the myelin sheath. J. Neurosci. 28, 6714–6719 (2008).
Feltri, M. L. et al. Conditional disruption of β1 integrin in Schwann cells impedes interactions with axons. J. Cell Biol. 156, 199–209 (2002).
Spassky, N. et al. Directional guidance of oligodendroglial migration by class 3 semaphorins and netrin-1. J. Neurosci. 22, 5992–6004 (2002).
Bannerman, P. et al. Peripheral nerve regeneration is delayed in neuropilin 2-deficient mice. J. Neurosci. Res. 86, 3163–3169 (2008).
Williams, A. et al. Semaphorin 3A and 3F: key players in myelin repair in multiple sclerosis? Brain 130, 2554–2565 (2007).
Moreau-Fauvarque, C. et al. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J. Neurosci. 23, 9229–9239 (2003).
Taniguchi, Y. et al. Sema4D deficiency results in an increase in the number of oligodendrocytes in healthy and injured mouse brains. J. Neurosci. Res. 87, 2833–2841 (2009).
Giraudon, P. et al. Semaphorin CD100 from activated T lymphocytes induces process extension collapse in oligodendrocytes and death of immature neural cells. J. Immunol. 172, 1246–1255 (2004).
Parrinello, S. et al. NF1 loss disrupts Schwann cell-axonal interactions: a novel role for semaphorin 4F. Genes Dev. 22, 3335–3348 (2008).
Manitt, C. et al. Widespread expression of netrin-1 by neurons and oligodendrocytes in the adult mammalian spinal cord. J. Neurosci. 21, 3911–3922 (2001).
Rajasekharan, S. et al. Netrin 1 and Dcc regulate oligodendrocyte process branching and membrane extension via Fyn and RhoA. Development 136, 415–426 (2009).
Jarjour, A. A. et al. Maintenance of axo-oligodendroglial paranodal junctions requires DCC and netrin-1. J. Neurosci. 28, 11003–11014 (2008).
Nakamoto, T., Kain, K. H. & Ginsberg, M. H. Neurobiology: new connections between integrins and axon guidance. Curr. Biol. 14, R121–R123 (2004).
Knoll, B. et al. Serum response factor controls neuronal circuit assembly in the hippocampus. Nat. Neurosci. 9, 195–204 (2006).
Wickramasinghe, S. R. et al. Serum response factor mediates NGF-dependent target innervation by embryonic DRG sensory neurons. Neuron 58, 532–545 (2008).
Stritt, C. et al. Paracrine control of oligodendrocyte differentiation by SRF-directed neuronal gene expression. Nat. Neurosci. 12, 418–427 (2009).
Monk, K. R. et al. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science 325, 1402–1405 (2009).
Chen, Y. et al. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat. Neurosci. 12, 1398–1406 (2009).
Lecca, D. et al. The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair. PLoS ONE 3, e3579 (2008).
Shen, S., Li, J. & Casaccia-Bonnefil, P. Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain. J. Cell Biol. 169, 577–589 (2005).
Ye, F. et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the β-catenin–TCF interaction. Nat. Neurosci. 12, 829–838 (2009).
He, Y. et al. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron 55, 217–230 (2007).
Shi, Y., Lee, J. S. & Galvin, K. M. Everything you have ever wanted to know about Yin Yang 1. Biochim. Biophys. Acta 1332, F49–F66 (1997).
Fancy, S. P. et al. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 23, 1571–1585 (2009).
Feigenson, K., Reid, M., See, J., Crenshaw, E. B. 3rd & Grinspan, J. B. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol. Cell. Neurosci. 42, 255–265 (2009).
Fu, H. et al. A genome-wide screen for spatially restricted expression patterns identifies transcription factors that regulate glial development. J. Neurosci. 29, 11399–11408 (2009).
Arnett, H. A. et al. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science 306, 2111–2115 (2004).
Xin, M. et al. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J. Neurosci. 25, 1354–1365 (2005).
Balabanov, R. & Popko, B. Myelin repair: developmental myelination redux? Nat. Neurosci. 8, 262–264 (2005).
Derfuss, T. et al. Contactin-2/TAG-1-directed autoimmunity is identified in multiple sclerosis patients and mediates gray matter pathology in animals. Proc. Natl Acad. Sci. USA 106, 8302–8307 (2009).
Passage, E. et al. Ascorbic acid treatment corrects the phenotype of a mouse model of Charcot-Marie-Tooth disease. Nat. Med. 10, 396–401 (2004).
Kaya, F et al. Ascorbic acid inhibits PMP22 expression by reducing cAMP levels. Neuromuscul. Disord. 17, 248–253 (2007).
Sereda, M. W., Meyer zu Hörste, G., Suter, U., Uzma, N. & Nave, K. A. Therapeutic administration of progesterone antagonist in a model of Charcot-Marie-Tooth disease (CMT-1A). Nat. Med. 9, 1533–1537 (2003).
Meyer zu Horste, G. et al. Antiprogesterone therapy uncouples axonal loss from demyelination in a transgenic rat model of CMT1A neuropathy. Ann. Neurol. 61, 61–72 (2007).
Pareyson, D. et al. A multicenter, randomized, double-blind, placebo-controlled trial of long-term ascorbic acid treatment in Charcot-Marie-Tooth disease type 1A (CMT-TRIAAL): the study protocol [EudraCT no.: 2006-000032-27]. Pharmacol. Res. 54, 436–441 (2006).
Shy, M. E. Therapeutic strategies for the inherited neuropathies. Neuromolecular Med. 8, 255–278 (2006).
Burns, J. et al. Ascorbic acid for Charcot-Marie-Tooth disease type 1A in children: a randomised, double-blind, placebo-controlled, safety and efficacy trial. Lancet Neurol. 8, 537–544 (2009).
Nave, K. A., Sereda, M. W. & Ehrenreich, H. Mechanisms of disease: inherited demyelinating neuropathies—from basic to clinical research. Nat. Clin. Pract. Neurol. 3, 453–464 (2007).
Fernandez, M. et al. Thyroid hormone administration enhances remyelination in chronic demyelinating inflammatory disease. Proc. Natl Acad. Sci. USA 101, 16363–16368 (2004).
Richardson, W. D., Kessaris, N. & Pringle, N. Oligodendrocyte wars. Nat. Rev. Neurosci. 7, 11–18 (2006).
Rorke, L. B. & Riggs, H. E. Myelination of the Brain in the Newborn (J. P. Lippincott Company, Philadelphia, 1969).
Birchmeier, C. ErbB receptors and the development of the nervous system. Exp. Cell Res. 315, 611–618 (2009).
Rosenberg, S. S., Ng, B. K. & Chan, J. R. The quest for remyelination: a new role for neurotrophins and their receptors. Brain Pathol. 16, 288–294 (2006).
Xiao, J. et al. BDNF exerts contrasting effects on peripheral myelination of NGF-dependent and BDNF-dependent DRG neurons. J. Neurosci. 29, 4016–4022 (2009).
Woolley, A. G. et al. Developmental loss of NT-3 in vivo results in reduced levels of myelin-specific proteins, a reduced extent of myelination and increased apoptosis of Schwann cells. Glia 56, 306–317 (2008).
Coman, I., Barbin, G., Charles, P., Zalc, B. & Lubetzki, C. Axonal signals in central nervous system myelination, demyelination and remyelination. J. Neurol. Sci. 233, 67–71 (2005).
Charles, P. et al. Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proc. Natl Acad. Sci. USA 97, 7585–7590 (2000).
Ishibashi, T. et al. Astrocytes promote myelination in response to electrical impulses. Neuron 49, 823–832 (2006).
Stevens, B. & Fields, R. D. Response of Schwann cells to action potentials in development. Science 287, 2267–2271 (2000).
Stevens, B., Ishibashi, T., Chen, J. F. & Fields, R. D. Adenosine: an activity-dependent axonal signal regulating MAP kinase and proliferation in developing Schwann cells. Neuron Glia Biol. 1, 23–34 (2004).
Feltri, M. L. & Wrabetz, L. Laminins and their receptors in Schwann cells and hereditary neuropathies. J. Peripher. Nerv. Syst. 10, 128–143 (2005).
Genoud, S., Maricic, I., Kumar, V. & Gage, F. H. Targeted expression of IGF-1 in the central nervous system fails to protect mice from experimental autoimmune encephalomyelitis. J. Neuroimmunol. 168, 40–45 (2005).
Schumacher, M., Sitruk-Ware, R. & De Nicola, A. F. Progesterone and progestins: neuroprotection and myelin repair. Curr. Opin. Pharmacol. 8, 740–746 (2008).
Park, S. K., Solomon, D. & Vartanian, T. Growth factor control of CNS myelination. Dev. Neurosci. 23, 327–337 (2001).
Franco, P. G., Silvestroff, L., Soto, E. F. & Pasquini, J. M. Thyroid hormones promote differentiation of oligodendrocyte progenitor cells and improve remyelination after cuprizone-induced demyelination. Exp. Neurol. 212, 458–467 (2008).
Denisenko, N. et al. Tumor suppressor schwannomin/merlin is critical for the organization of Schwann cell contacts in peripheral nerves. J. Neurosci. 28, 10472–10481 (2008).
Hoke, A. & Keswani, S. C. Neuroprotection in the PNS: erythropoietin and immunophilin ligands. Ann. NY Acad. Sci. 1053, 491–501 (2005).
Werner, H. B. et al. Proteolipid protein is required for transport of sirtuin 2 into CNS myelin. J. Neurosci. 27, 7717–7730 (2007).
Furusho, M., Dupree, J. L., Bryant, M. & Bansal, R. Disruption of fibroblast growth factor receptor signaling in nonmyelinating Schwann cells causes sensory axonal neuropathy and impairment of thermal pain sensitivity. J. Neurosci. 29, 1608–1614 (2009).
Verheijen, M. H. et al. SCAP is required for timely and proper myelin membrane synthesis. Proc. Natl Acad. Sci. USA 106, 21383–21388 (2009).
Inoue, K. et al. Congenital hypomyelinating neuropathy, central dysmyelination, and Waardenburg-Hirschsprung disease: phenotypes linked by SOX10 mutation. Ann. Neurol. 52, 836–842 (2002).
Acknowledgements
Work in the laboratories of C. Taveggia, M. L. Feltri and L. Wrabetz is supported by grants from Fondazione Italiana Sclerosi Multipla, Italy; Telethon, Italy; Compagnia di San Paolo, Italy; Fondazione Mariani, Italy; the NIH, USA; and the European Union. We apologize to colleagues whose relevant work we were unable to cite because of space limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Taveggia, C., Feltri, M. & Wrabetz, L. Signals to promote myelin formation and repair. Nat Rev Neurol 6, 276–287 (2010). https://doi.org/10.1038/nrneurol.2010.37
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2010.37
This article is cited by
-
The Schwann cell-specific G-protein Gαo (Gnao1) is a cell-intrinsic controller contributing to the regulation of myelination in peripheral nerve system
Acta Neuropathologica Communications (2024)
-
Transcriptome Analysis of Schwann Cells at Various Stages of Myelination Implicates Chromatin Regulator Sin3A in Control of Myelination Identity
Neuroscience Bulletin (2022)
-
Icariin Ameliorates Alzheimer’s Disease Pathology by Alleviating Myelin Injury in 3 × Tg-AD Mice
Neurochemical Research (2022)
-
White matter injury in infants with intraventricular haemorrhage: mechanisms and therapies
Nature Reviews Neurology (2021)
-
Advances for the Development of In Vitro Immunosensors for Multiple Sclerosis Diagnosis
BioChip Journal (2021)