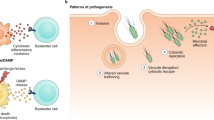
Abstract
A fundamental question regarding any immune system is how it can discriminate between pathogens and non-pathogens. Here, we discuss how this discrimination can be mediated by a surveillance system distinct from pattern-recognition receptors that recognize conserved microbial patterns. It can be based instead on the ability of the host to sense perturbations in host cells induced by bacterial toxins or 'effectors' that are encoded by pathogenic microorganisms. Such 'effector-triggered immunity' was previously demonstrated mainly in plants, but recent data confirm that animals can also use this strategy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Janeway, C. A. Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54, 1–13 (1989).
Medzhitov, R. Approaching the asymptote: 20 years later. Immunity 30, 766–775 (2009).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature Immunol. 11, 373–384 (2010).
Kau, A. L., Ahern, P. P., Griffin, N. W., Goodman, A. L. & Gordon, J. I. Human nutrition, the gut microbiome and the immune system. Nature 474, 327–336 (2011).
Medzhitov, R. Innate immunity: quo vadis? Nature Immunol. 11, 551–553 (2010).
Hooper, L. V. & Macpherson, A. J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nature Rev. Immunol. 10, 159–169 (2010).
Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045 (1994).
Matzinger, P. An innate sense of danger. Semin. Immunol. 10, 399–415 (1998).
Matzinger, P. The danger model: a renewed sense of self. Science 296, 301–305 (2002).
Shi, Y., Evans, J. E. & Rock, K. L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425, 516–521 (2003).
Scaffidi, P., Misteli, T. & Bianchi, M. E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195 (2002).
Vance, R. E., Isberg, R. R. & Portnoy, D. A. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe 6, 10–21 (2009).
Sauer, J. D. et al. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe 7, 412–419 (2010).
Abreu, M. T. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nature Rev. Immunol. 10, 131–144 (2010).
Sander, L. E. et al. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature 474, 385–389 (2011).
Blander, J. M. & Sander, L. E. Beyond pattern recognition: five immune checkpoints for scaling the microbial threat. Nature Rev. Immunol. 12, 215–225 (2012).
Fontana, M. F. & Vance, R. E. Two signal models in innate immunity. Immunol. Rev. 243, 26–39 (2011).
Flor, H. Inheritance of pathogenicity in Melampsora lini. Phytopathology 32, 653–669 (1942).
Flor, H. Current status of the gene-for-gene concept. Annu. Rev. Phytopathol. 9, 275–296 (1971).
Staskawicz, B. J., Dahlbeck, D. & Keen, N. T. Cloned avirulence gene of Pseudomonas syringae pv. glycinea determines race-specific incompatibility on Glycine max (L.) Merr. Proc. Natl Acad. Sci. USA 81, 6024–6028 (1984).
Chisholm, S. T., Coaker, G., Day, B. & Staskawicz, B. J. Host–microbe interactions: shaping the evolution of the plant immune response. Cell 124, 803–814 (2006).
Jones, J. D. & Dangl, J. L. The plant immune system. Nature 444, 323–329 (2006).
Dangl, J. L. & Jones, J. D. Plant pathogens and integrated defence responses to infection. Nature 411, 826–833 (2001).
Bruno, V. M. et al. Salmonella Typhimurium type III secretion effectors stimulate innate immune responses in cultured epithelial cells. PLoS Pathog. 5, e1000538 (2009).
Brandt, S. M. et al. Secreted bacterial effectors and host-produced Eiger/TNF drive death in a Salmonella-infected fruit fly. PLoS Biol. 2, e418 (2004).
Boyer, L. et al. Pathogen-derived effectors trigger protective immunity via activation of the Rac2 enzyme and the IMD or Rip kinase signaling pathway. Immunity 35, 536–549 (2011).
Fontana, M. F. et al. Secreted bacterial effectors that inhibit host protein synthesis are critical for induction of the innate immune response to virulent Legionella pneumophila. PLoS Pathog. 7, e1001289 (2011).
Fontana, M. F., Shin, S. & Vance, R. E. Activation of host mitogen-activated protein kinases by secreted Legionella pneumophila effectors that inhibit host protein translation. Infect. Immun. 80, 3570–3575 (2012).
Dunbar, T. L., Yan, Z., Balla, K. M., Smelkinson, M. G. & Troemel, E. R. C. elegans detects pathogen-induced translational inhibition to activate immune signaling. Cell Host Microbe 11, 375–386 (2012).
McEwan, D. L., Kirienko, N. V. & Ausubel, F. M. Host translational inhibition by Pseudomonas aeruginosa exotoxin A triggers an immune response in Caenorhabditis elegans. Cell Host Microbe 11, 364–374 (2012).
Casadevall, A. & Pirofski, L. A. The damage-response framework of microbial pathogenesis. Nature Rev. Microbiol. 1, 17–24 (2003).
Pirofski, L. A. & Casadevall, A. The damage-response framework of microbial pathogenesis and infectious diseases. Adv. Exp. Med. Biol. 635, 135–146 (2008).
Galan, J. E. Common themes in the design and function of bacterial effectors. Cell Host Microbe 5, 571–579 (2009).
Mattoo, S., Lee, Y. M. & Dixon, J. E. Interactions of bacterial effector proteins with host proteins. Curr. Opin. Immunol. 19, 392–401 (2007).
Ribet, D. & Cossart, P. Pathogen-mediated posttranslational modifications: a re-emerging field. Cell 143, 694–702 (2010).
Brodsky, I. E. & Medzhitov, R. Targeting of immune signalling networks by bacterial pathogens. Nature Cell Biol. 11, 521–526 (2009).
Gottar, M. et al. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell 127, 1425–1437 (2006).
Ausubel, F. M. Are innate immune signaling pathways in plants and animals conserved? Nature Immunol. 6, 973–979 (2005).
Dodds, P. N. & Rathjen, J. P. Plant immunity: towards an integrated view of plant–pathogen interactions. Nature Rev. Genet. 11, 539–548 (2010).
Gomez-Gomez, L. & Boller, T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell 5, 1003–1011 (2000).
Hayashi, F. et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103 (2001).
Shin, S. et al. Type IV secretion-dependent activation of host MAP kinases induces an increased proinflammatory cytokine response to Legionella pneumophila. PLoS Pathog. 4, e1000220 (2008).
Bartfeld, S. et al. Temporal resolution of two-tracked NF-κB activation by Legionella pneumophila. Cell. Microbiol. 11, 1638–1651 (2009).
Aroian, R. & van der Goot, F. G. Pore-forming toxins and cellular non-immune defenses (CNIDs). Curr. Opin. Microbiol. 10, 57–61 (2007).
Freche, B., Reig, N. & van der Goot, F. G. The role of the inflammasome in cellular responses to toxins and bacterial effectors. Semin. Immunopathol. 29, 249–260 (2007).
Gurcel, L., Abrami, L., Girardin, S., Tschopp, J. & van der Goot, F. G. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126, 1135–1145 (2006).
Huffman, D. L. et al. Mitogen-activated protein kinase pathways defend against bacterial pore-forming toxins. Proc. Natl Acad. Sci. USA 101, 10995–11000 (2004).
Bebien, M. et al. The pore-forming toxin β hemolysin/cytolysin triggers p38 MAPK-dependent IL-10 production in macrophages and inhibits innate immunity. PLoS Pathog. 8, e1002812 (2012).
Porta, H., Cancino-Rodezno, A., Soberon, M. & Bravo, A. Role of MAPK p38 in the cellular responses to pore-forming toxins. Peptides 32, 601–606 (2011).
Kao, C. Y. et al. Global functional analyses of cellular responses to pore-forming toxins. PLoS Pathog. 7, e1001314 (2011).
Husmann, M. et al. Elimination of a bacterial pore-forming toxin by sequential endocytosis and exocytosis. FEBS Lett. 583, 337–344 (2009).
Idone, V. et al. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol. 180, 905–914 (2008).
Medzhitov, R., Schneider, D. S. & Soares, M. P. Disease tolerance as a defense strategy. Science 335, 936–941 (2012).
Meinzer, U. et al. Yersinia pseudotuberculosis effector YopJ subverts the Nod2/RICK/TAK1 pathway and activates caspase-1 to induce intestinal barrier dysfunction. Cell Host Microbe 11, 337–351 (2012).
Mittal, R., Peak-Chew, S. Y. & McMahon, H. T. Acetylation of MEK2 and IκB kinase (IKK) activation loop residues by YopJ inhibits signaling. Proc. Natl Acad. Sci. USA 103, 18574–18579 (2006).
Mukherjee, S. et al. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 312, 1211–1214 (2006).
Paquette, N. et al. Serine/threonine acetylation of TGFβ-activated kinase (TAK1) by Yersinia pestis YopJ inhibits innate immune signaling. Proc. Natl Acad. Sci. USA 109, 12710–12715 (2012).
Hann, D. & Boller, T. in Effectors in Plant–Microbe Interactions (eds Martin, F. & Kamoun, S.) 33–52 (Wiley-Blackwell, 2011).
Acknowledgements
The authors would like to thank E. Lemichez, F. Ausubel, A. Lacy-Hulbert and J. Irazoqui for their helpful discussion and critical reading of the manuscript. N.P. was supported by the US National Institutes of Health (NIH)/US National Institute of Allergy and Infectious Diseases (NIAID) through the New England Regional Center of Excellence (NERCE) (U54 AI057159); L.B. was supported by Ligue Nationale Contre le Cancer, by institutional funding from INSERM and by grants from Fondation Infectiopôle Sud and Fondation ARC pour la Recherche sur le Cancer (ARC SFI 20111203659 and ARC SFI 20121205382); and L.M.S. was supported by grants from NIH/NIAID.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Avirulence gene
-
(Avr gene). The original plant terminology for genes encoding bacterial effectors that induce effector-triggered immunity in the resistant host.
- Damage-associated molecular patterns
-
(DAMPs). Self-derived immune elicitors that indicate damage to the host. As a result of cellular stress, cellular damage or non-physiological cell death, DAMPs are released from various compartments, including the degraded stroma (in the case of hyaluronate, for example), the nucleus (in the case of high-mobility group protein B1) and the cytosol (in the case of ATP, uric acid, S100 calcium-binding proteins and heat-shock proteins). Such DAMPs are thought to elicit local inflammatory reactions.
- Effector-triggered immune pathology
-
(ETIP). An over-exuberant immune response induced by bacterial effectors that benefits the pathogen and is to the detriment of the host.
- Effector-triggered immunity
-
(ETI). The detection of and response to microbial effectors that enter the host cell and trigger a protective immune response by the host.
- Effector-triggered susceptibility
-
(ETS). Immune suppression or evasion caused by bacterial effectors that enter the host cell and disrupt defence pathways.
- IMD innate immune signalling pathway
-
One of two innate immune NF-κB signalling pathways in Drosophila melanogaster. The IMD pathway responds to DAP-type peptidoglycan from Gram-negative, and some Gram-positive, bacteria. This leads to the rapid and robust production of antimicrobial peptides.
- Microorganism-associated molecular patterns
-
(MAMPs). After the realization that PAMPs are found in both pathogens and non-pathogens, the term MAMPs was suggested as a more appropriate description of the microbial ligands that bind to PRRs.
- Pathogen-associated molecular patterns
-
(PAMPs). The term originally used to describe the immune elicitors found in bacteria, but not in mammalian cells, that bind to PRRs and drive immune responses. Examples include terminally mannosylated and polymannosylated compounds, which bind to the mannose receptor, and various microbial products, such as bacterial lipopolysaccharides, hypomethylated DNA, flagellin and double-stranded RNA, which bind to Toll-like receptors. This term has now been superseded by MAMPs.
- Pattern-recognition receptors
-
(PRRs). Proteins expressed by innate immune cells that detect molecules associated with microbial pathogens or cellular stress.
- Pattern-triggered immunity
-
(PTI). The immune response elicited after the ligation of a PRR by its microbial ligand. A more appropriate term would in fact be MAMP-triggered immunity, for the reasons described above.
- Resistance gene
-
(R gene). The original plant terminology for a host gene encoding a protein that is required for the direct or indirect sensing of a pathogen effector and that elicits a protective immune response.
- Sterile inflammation
-
Inflammation triggered by the danger signals that are induced by chemical or physical stress, in the absence of infection.
- Type III secretion system
-
A specialized molecular machine present in some bacteria that allows the injection of bacterial proteins into host cells.
- 'Zig-zag' model
-
A model that was proposed to illustrate the quantitative output of the plant immune system and to explain the co-evolution of plant resistance genes and pathogen effectors.
Rights and permissions
About this article
Cite this article
Stuart, L., Paquette, N. & Boyer, L. Effector-triggered versus pattern-triggered immunity: how animals sense pathogens. Nat Rev Immunol 13, 199–206 (2013). https://doi.org/10.1038/nri3398
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3398
This article is cited by
-
Unconventional secretion of Magnaporthe oryzae effectors in rice cells is regulated by tRNA modification and codon usage control
Nature Microbiology (2023)
-
Lysosome-related organelles promote stress and immune responses in C. elegans
Communications Biology (2023)
-
Recent advances in mRNA-LNP therapeutics: immunological and pharmacological aspects
Journal of Nanobiotechnology (2022)
-
C. elegans monitor energy status via the AMPK pathway to trigger innate immune responses against bacterial pathogens
Communications Biology (2022)
-
Escherichia coli Rho GTPase-activating toxin CNF1 mediates NLRP3 inflammasome activation via p21-activated kinases-1/2 during bacteraemia in mice
Nature Microbiology (2021)