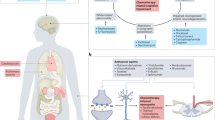
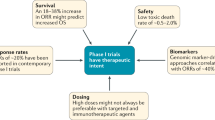
Abstract
Our understanding of the biology of cancer and the application of this knowledge to cancer treatment has greatly outpaced what we know of the biology underlying the symptoms and toxic effects that therapies produce. These adverse effects of therapy cause substantial discomfort and distress to patients and their families, limit treatment tolerability and can persist indefinitely in post-treatment survivorship. Despite these concerns, little research effort is targeted at documenting the nature of these effects. Similarly, limited efforts are being made in the drug-development arena to identify or develop treatments that might prevent or reduce toxicities. A panel of clinicians and researchers as well as representatives from advocacy groups, federal agencies and the pharmaceutical industry was convened to identify gaps in cancer treatment toxicity research and to provide direction for future action. With an emphasis on coordinating multidisciplinary efforts, this panel has presented a strategy to increase funding for the field and develop a coherent research agenda.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Centers for Disease Control and Prevention (CDC). Cancer survivors—United States, 2007. MMWR Morb. Mortal. Wkly Rep. 60, 269–272 (2011).
Dantzer, R., Meagher, M. W. & Cleeland, C. S. Translational approaches to treatment-induced symptoms in cancer patients. Nat. Rev. Clin. Oncol. http://dx.doi.org/10.1038/nrclinonc.2012.88
Segota, E. & Bukowski, R. M. The promise of targeted therapy: cancer drugs become more specific. Cleve. Clin. J. Med. 71, 551–560 (2004).
Fakih, M. & Vincent, M. Adverse events associated with anti-EGFR therapies for the treatment of metastatic colorectal cancer. Curr. Oncol. 17 (Suppl. 1), S18–S30 (2010).
Sankhala, K. et al. The emerging safety profile of mTOR inhibitors, a novel class of anticancer agents. Target. Oncol. 4, 135–142 (2009).
Shepard, D. R. & Garcia, J. A. Toxicity associated with the long-term use of targeted therapies in patients with advanced renal cell carcinoma. Expert Rev. Anticancer Ther. 9, 795–805 (2009).
Subbiah, I. M., Lenihan, D. J. & Tsimberidou, A. M. Cardiovascular toxicity profiles of vascular-disrupting agents. Oncologist 16, 1120–1130 (2011).
Yang, X. et al. Kinase inhibition-related adverse events predicted from in vitro kinome and clinical trial data. J. Biomed. Inform. 43, 376–384 (2010).
Bostrom, P. J. & Soloway, M. S. Secondary cancer after radiotherapy for prostate cancer: should we be more aware of the risk? Eur. Urol. 52, 973–982 (2007).
Chaturvedi, A. K. et al. Second cancers among 104,760 survivors of cervical cancer: evaluation of long-term risk. J. Natl Cancer Inst. 99, 1634–1643 (2007).
Kry, S. F. et al. The calculated risk of fatal secondary malignancies from intensity-modulated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 62, 1195–1203 (2005).
Nieder, A. M., Porter, M. P. & Soloway, M. S. Radiation therapy for prostate cancer increases subsequent risk of bladder and rectal cancer: a population based cohort study. J. Urol. 180, 2005–2009 (2008).
Smith, R. E., Bryant, J., DeCillis, A. & Anderson, S. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J. Clin. Oncol. 21, 1195–1204 (2003).
Basch, E. et al. Symptom measurement in clinical trials. Conference on Clinical Cancer Research, November 2011 [online], (2011).
Movsas, B. et al. Decreasing the adverse effects of cancer therapy: National Cancer Institute guidance for the clinical development of radiation injury mitigators. Clin. Cancer Res. 17, 222–228 (2011).
Smith, T. J. et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J. Clin. Oncol. 30, 880–887 (2012).
Rose, D. E. et al. Prevalence, predictors, and patient outcomes associated with physician co-management: findings from the Los Angeles Women's Health Study. Health Serv. Res. 47, 1091–1116 (2012).
FDA. Drugs: Adverse Event Reporting System (AERS) [online], (2012).
Institute of Medicine (US) Forum on Drug Discovery, Development, and Translation. Emerging safety science: workshop summary [online], (2008).
FDA. Safety: FDA's Sentinel Initiative [online], (2012).
American College of Surgeons. Cancer programs: national cancer data base [online], (2011).
National Cancer Institute. Surveillance Epidemiology and End Results [online].
Centers for Disease Control and Prevention. National Program of Cancer Registries (NPCR) [online], (2012).
Abernethy, A. P. et al. Rapid-learning system for cancer care. J. Clin. Oncol. 28, 4268–4274 (2010).
National Cancer Institute. Factsheet: NCI's clinical trials cooperative group program [online], (2012).
PatientsLikeMe® [online], (2012).
Wicks, P., Vaughan, T. E., Massagli, M. P. & Heywood, J. Accelerated clinical discovery using self-reported patient data collected online and a patient-matching algorithm. Nat. Biotechnol. 29, 411–414 (2011).
Oeffinger, K. C. et al. Chronic health conditions in adult survivors of childhood cancer. N. Engl. J. Med. 355, 1572–1582 (2006).
ADNI Alzheimer's Disease Neuroimaging Initiative [online], (2012).
Lacouture, M. E. et al. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support. Care Cancer 19, 1079–1095 (2011).
Busaidy, N. L. et al. Management of metabolic effects (hyperlipidemia and hyperglycemia) associated with anticancer agents targeting the pi3k-Akt-mTOR (PAM) pathway. J. Clin. Oncol. (in press).
National Comprehensive Cancer Institute. NCCN Guidelines & Clinical Resources: NCCN guidelines for supportive care [online].
Ettinger, D. S. et al. Antiemesis clinical practice guidelines in oncology. J. Natl Compr. Canc. Netw. 7, 572–595 (2009).
Rodgers, G. M. III. et al. Cancer- and chemotherapy-induced anemia. J. Natl Compr. Canc. Netw. 10, 628–653 (2012).
Basch, E. et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 29, 4189–4198 (2011).
Hensley, M. L. et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J. Clin. Oncol. 27, 127–145 (2009).
Lyman, G. H. et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J. Clin. Oncol. 25, 5490–5505 (2007).
Eagle, D. & Sprandio, J. A care model for the future: the oncology medical home. Oncology (Williston Park) 25, 571–576 (2011).
National Cancer Research Institute. Rapid review of research in survivorship after cancer and end of life care [online] (2010).
National Cancer Institute. Division of Cancer Prevention programs & resources: Community Clinical Oncology Program (CCOP) [online].
National Cancer Institute. Cancer survivorship research: cancer control and population sciences [online], (2012).
Hoofnagle, J. H. Drug-induced liver injury network (DILIN). Hepatology 40, 773 (2004).
Stang, P. E. et al. Advancing the science for active surveillance: rationale and design for the Observational Medical Outcomes Partnership. Ann. Intern. Med. 153, 600–606 (2010).
International Conference on Harmonisation (ICH). Safety guidelines [online], (2012).
Olson, H. et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 32, 56–67 (2000).
Seigers, R. & Fardell, J. E. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci. Biobehav. Rev. 35, 729–741 (2011).
Bowen, J. M., Gibson, R. J. & Keefe, D. M. Animal models of mucositis: implications for therapy. J. Support. Oncol. 9, 161–168 (2011).
Walker, E. A. Animal models. Adv. Exp. Med. Biol. 678, 138–146 (2010).
Meagher, M. W. in Cancer Symptom Science: Measurement, Mechanisms, and Management (eds Cleeland, C. S., Fisch, M. J. & Dunn, A. J.) 124–141 (Cambridge University Press, Cambridge UK, 2011).
Clement, C. G. et al. Stimulation of lung innate immunity protects against lethal pneumococcal pneumonia in mice. Am. J. Respir. Crit. Care Med. 177, 1322–1330 (2008).
Force, T. & Kolaja, K. L. Cardiotoxicity of kinase inhibitors: the prediction and translation of preclinical models to clinical outcomes. Nat. Rev. Drug Discov. 10, 111–126 (2011).
Zhang, H., Yoon, S. Y., Zhang, H. & Dougherty, P. M. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of paclitaxel-induced painful neuropathy. J. Pain 13, 293–303 (2012).
Mandenius, C. F. et al. Cardiotoxicity testing using pluripotent stem cell-derived human cardiomyocytes and state-of-the-art bioanalytics: a review. J. Appl. Toxicol. 31, 191–205 (2011).
Turteltaub, K. W. et al. Identification and elucidation of the biology of adverse events: the challenges of safety assessment and translational medicine. Clin. Cancer Res. 17, 6641–6645 (2011).
Abernethy, D. R., Woodcock, J. & Lesko, L. J. Pharmacological mechanism-based drug safety assessment and prediction. Clin. Pharmacol. Ther. 89, 793–797 (2011).
Reagan–Udall Foundation for the FDA. Projects [online], (2012).
Basch, E. et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J. Natl. Cancer Inst. 101, 1624–1632 (2009).
Basch, E. M. et al. Electronic toxicity monitoring and patient-reported outcomes. Cancer J. 17, 231–234 (2011).
US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research & Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims [online], (2009).
Dempke, W. C., Suto, T. & Reck, M. Targeted therapies for non-small cell lung cancer. Lung Cancer 67, 257–274 (2010).
Yap, T. A. & Workman, P. Exploiting the cancer genome: strategies for the discovery and clinical development of targeted molecular therapeutics. Annu. Rev. Pharmacol. Toxicol. 52, 549–573 (2012).
Yeung, D. T. & Hughes, T. P. Therapeutic targeting of BCR-ABL: prognostic markers of response and resistance mechanism in chronic myeloid leukaemia. Crit. Rev. Oncog. 17, 17–30 (2012).
Brooks, P. et al. OMERACT 10—International consensus conference on outcome measures in rheumatology clinical trials. J. Rheumatol. 38, 1450–1451 (2011).
Wortel, C. H. et al. Interleukin-6 mediates host defense responses induced by abdominal surgery. Surgery 114, 564–570 (1993).
Kerawala, C. J. Complications of head and neck cancer surgery - prevention and management. Oral Oncol. 46, 433–435 (2010).
Williams, J. P., Johnston, C. J. & Finkelstein, J. N. Treatment for radiation-induced pulmonary late effects: spoiled for choice or looking in the wrong direction? Curr. Drug Targets 11, 1386–1394 (2010).
Abratt, R. P. & Morgan, G. W. Lung toxicity following chest irradiation in patients with lung cancer. Lung Cancer 35, 103–109 (2002).
Armstrong, T. & Gilbert, M. R. Central nervous system toxicity from cancer treatment. Curr. Oncol. Rep. 6, 11–19 (2004).
Kim, J. H., Brown, S. L., Jenrow, K. A. & Ryu, S. Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J. Neurooncol. 87, 279–286 (2008).
Perry, A. & Schmidt, R. E. Cancer therapy-associated CNS neuropathology: an update and review of the literature. Acta Neuropathol. 111, 197–212 (2006).
Sonis, S. T. Regimen-related gastrointestinal toxicities in cancer patients. Curr. Opin. Support. Palliat. Care 4, 26–30 (2010).
Sul, J. K. & DeAngelis, L. M. Neurologic complications of cancer chemotherapy. Semin. Oncol. 33, 324–332 (2006).
Cleeland, C. S., Farrar, J. T. & Hausheer, F. H. Assessment of cancer-related neuropathy and neuropathic pain. Oncologist. 15 (Suppl. 2), 13–18 (2010).
Hagiwara, H. & Sunada, Y. Mechanism of taxane neurotoxicity. Breast Cancer 11, 82–85 (2004).
Mohty, B. et al. Peripheral neuropathy and new treatments for multiple myeloma: background and practical recommendations. Haematologica 95, 311–319 (2010).
Albini, A. et al. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J. Natl Cancer Inst. 102, 14–25 (2010).
Curigliano, G., Mayer, E. L., Burstein, H. J., Winer, E. P. & Goldhirsch, A. Cardiac toxicity from systemic cancer therapy: a comprehensive review. Prog. Cardiovasc. Dis. 53, 94–104 (2010).
Daher, I. N. & Yeh, E. T. Vascular complications of selected cancer therapies. Nat. Clin. Pract. Cardiovasc. Med. 5, 797–805 (2008).
Ahn, S. & Lee, Y. S. Predictive factors for poor prognosis febrile neutropenia. Curr. Opin. Oncol. http://dx.doi./org/10.1097/CCO.0b013e328352ead2 (2012).
Greenspan, S. L. et al. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J. Clin. Endocrinol. Metab. 90, 6410–6417 (2005).
Krychman, M. L. & Katz, A. Breast cancer and sexuality: multi-modal treatment options. J. Sex. Med. 9, 5–25 (2012).
Di Lorenzo, G. et al. Toxicities of targeted therapy and their management in kidney cancer. Eur. Urol. 59, 526–540 (2011).
Force, T. & Kerkelä, R. Cardiotoxicity of the new cancer therapeutics—mechanisms of, and approaches to, the problem. Drug Discov. Today 13, 778–784 (2008).
Tesfa, D. & Palmblad, J. Late-onset neutropenia following rituximab therapy: incidence, clinical features and possible mechanisms. Expert Rev. Hematol. 4, 619–625 (2011).
Acknowledgements
The authors thank Tim Ahles (American Association for Cancer Research Survivorship Task Force, New York, NY, USA), Ann O'Mara (National Cancer Institute [NCI], Bethesda, MD, USA), Jamie H. Von Roenn (ASCO, Chicago, IL, USA), Elaine B. Yu (Genentech, San Francisco, CA, USA) and Nora Janjan (National Center for Policy Analysis, Dallas, TX, USA) for their insightful comments during the drafting of this article. We also acknowledge Jeanie F. Woodruff for editorial assistance, and M. Catherine Rodgers for administrative assistance (both at The University of Texas MD Anderson Cancer Center, Houston, TX, USA). The authors wish to acknowledge the overall contribution of those who participated in and contributed to the March 2011 colloquium entitled Developing Strategies for Reducing Cancer Treatment-Related Toxicities and Symptoms in Houston, TX, USA: Lyndah K. Dreiling (Amgen, Thousand Oaks, CA, USA), Marilee Duffield (Alere Health, Marietta, GA, USA), Robert F. Gagel (The University of Texas MD Anderson Cancer Center, Houston, TX, USA), Mark Gorman (National Coalition for Cancer Survivorship, Silver Spring, MD, USA), Amy Guo (Novartis, East Hanover, NJ, USA), Carol A. Hahn (American Society for Therapeutic Radiation Oncology, Durham, NC, USA), Joan S. McClure (National Comprehensive Cancer Network, Fort Washington, PA, USA), Andrew Miller (Lance Armstrong Foundation, Austin, TX, USA), Scarlott K. Mueller (Oncology Nursing Society Foundation, Pittsburgh, PA, USA), Robert Z. Orlowski (The University of Texas MD Anderson Cancer Center, Houston, TX, USA), Lorna Patrick (NCI, Rockville, MD, USA), Ellen B. Smith (Texas Oncology, Austin, TX, USA), Mark Stephens (National Patient Advocate Foundation, Washington, DC, USA), Steve G. Waguespack (The University of Texas MD Anderson Cancer Center, Houston, TX, USA), Robert A. Warriner III (patient advocate and Diversified Clinical Services, Spring, TX, USA) and Armin D. Weinberg (Baylor College of Medicine, Houston, TX, USA). Funding support for the meeting was provided by the C. Stratton Hill Colloquium on Pain and Its Relief. The opinions expressed in this article are those of the panellists and do not constitute a policy position of the NIH, National Cancer Institute, US Department of Health and Human Services or the US government.
Author information
Authors and Affiliations
Contributions
All authors participated in the colloquium, contributed to the discussion of the article content and took part in the initial drafting of the article, after which C. S. Cleeland and S. A. Roberts wrote the full manuscript. All authors edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
C. S. Cleeland has acted as a consultant or advisor for Abbott, Amgen, Bristol–Myers Squibb, Exelixis, Genentech and Pfizer and has received research funding from AstraZeneca. S. A. Giralt has acted as a consultant or advisor for Celgene, Novartis and Millennium and has received funding from Celgene. A. Y. Khakoo is employed by and owns stock in Amgen. J. Skillings is employed by Pfizer. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Cleeland, C., Allen, J., Roberts, S. et al. Reducing the toxicity of cancer therapy: recognizing needs, taking action. Nat Rev Clin Oncol 9, 471–478 (2012). https://doi.org/10.1038/nrclinonc.2012.99
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2012.99
This article is cited by
-
Omega-3 fatty acids as adjuvant therapy in the adverse effects of antineoplastic treatment for breast cancer: a systematic review
Nutrire (2023)
-
Biodegradable FePS3 nanoplatform for efficient treatment of osteosarcoma by combination of gene and NIR-II photothermal therapy
Journal of Nanobiotechnology (2023)
-
Patient-centered dosing: oncologists’ perspectives about treatment-related side effects and individualized dosing for patients with metastatic breast cancer (MBC)
Breast Cancer Research and Treatment (2022)
-
MOFs-based nanoagent enables dual mitochondrial damage in synergistic antitumor therapy via oxidative stress and calcium overload
Nature Communications (2021)
-
Patient-reported outcomes in light of supportive medications in treatment-naïve lung cancer patients
Supportive Care in Cancer (2020)